Abstract
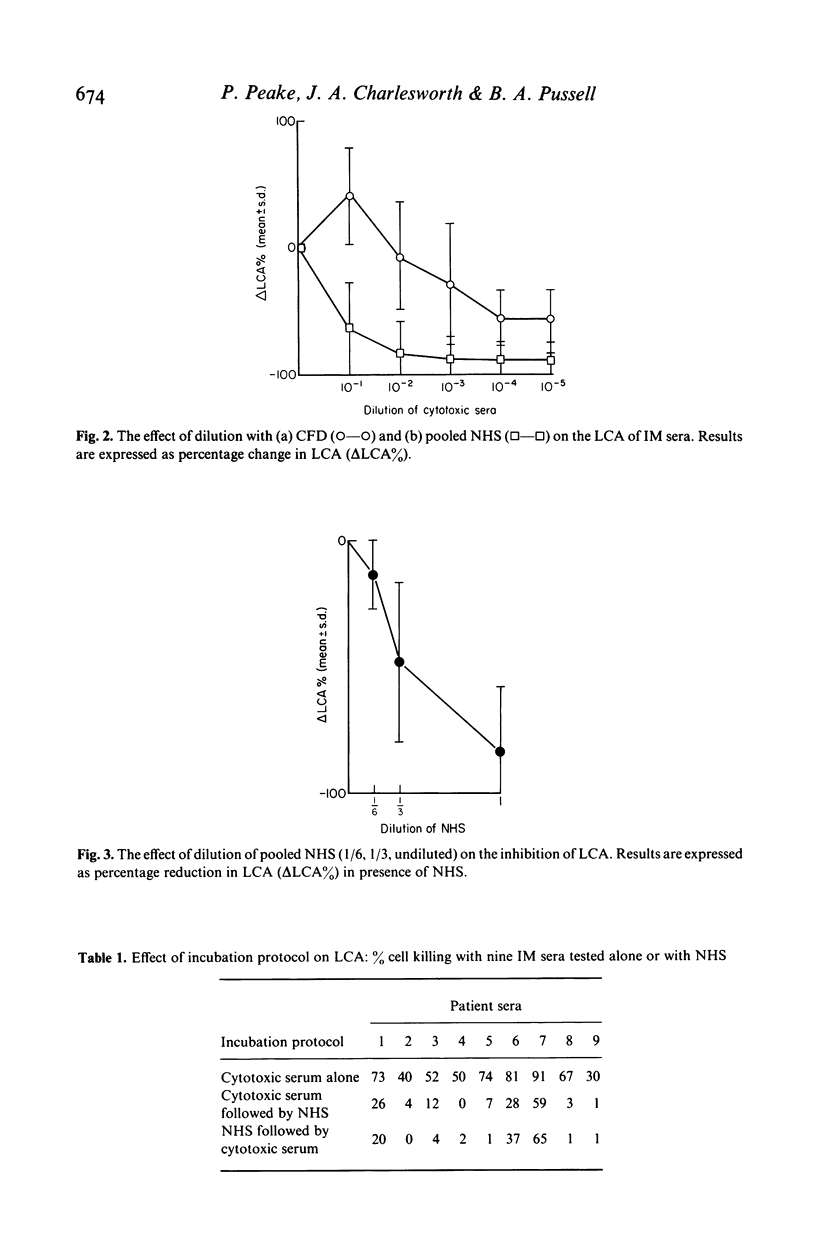
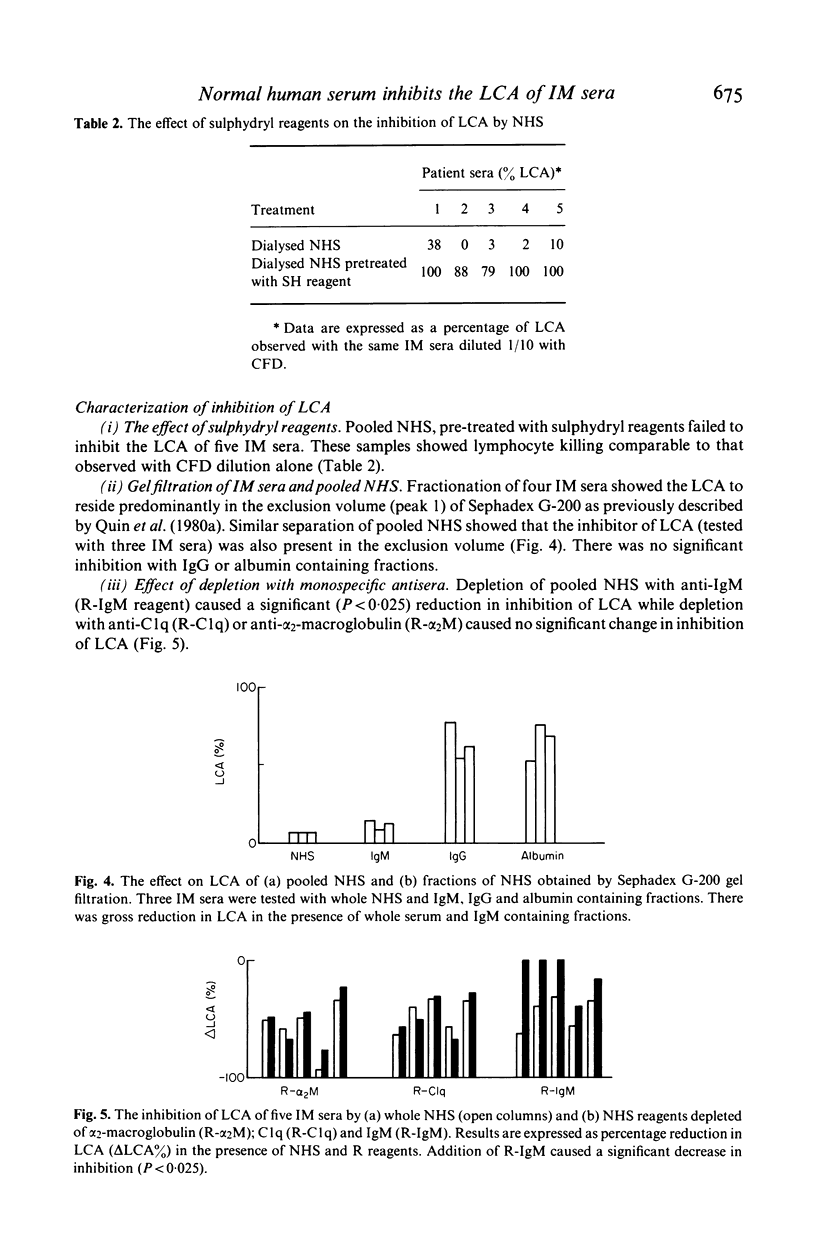
The inhibitory effects of normal human serum (NHS) on the lymphocytotoxic activity (LCA) of sera from patients with infectious mononucleosis (IM) were investigated. Dilution of IM serum with complement fixation diluent (CFD) caused a significant rise in LCA at 1/10 dilution (P less than 0.001) followed by a steady decline at higher dilutions. In contrast, 1/10 dilution with pooled NHS caused a gross reduction in lymphocyte killing (P less than 0.01). This reduction occurred irrespective of the order of incubation of NHS and IM serum with target lymphocytes. Pre-dilution of the NHS showed this inhibitory effect to be dose-responsive. Further characterization of the inhibitor(s) showed it to reside in the exclusion peak of Sephadex G-200, to be abolished by treatment with the sulphydryl inhibitors, 2-mercaptoethanol and iodoacetamide and to be depleted selectively by incubation with monospecific anti-IgM (but not anti-C1q or anti-alpha 2-macroglobulin). The site of the inhibitory reaction was examined by indirect immunoperoxidase staining of the lymphocyte surface with monospecific anti-IgM and peroxidase conjugated swine anti-rabbit immunoglobulin. This showed that pre-incubation of IM serum with NHS caused a significant reduction in IgM positive cells compared to that observed with IM serum diluted in CFD alone. It is concluded that certain IgM molecules, present in NHS, inhibit the complement-mediated LCA of IM sera. This inhibition occurs by fluid phase interference with surface deposition of cytotoxic IgM rather than by competitive surface binding. The presence of such serum-serum interactions emphasises the complexity of the lymphocytotoxin reaction and the need for caution in attributing abnormalities in vivo to cytotoxic phenomena measured in vitro.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cicciarelli J. C., Chia D., Terasaki P. I., Barnett E. V., Shirahama S. IgM anti-IgM cold lymphocytotoxins to B cells. Transplant Proc. 1979 Dec;11(4):1950–1953. [PubMed] [Google Scholar]
- De Waele M., Thielemans C., Van Camp B. K. Characterization of immunoregulatory T cells in EBV-induced infectious mononucleosis by monoclonal antibodies. N Engl J Med. 1981 Feb 19;304(8):460–462. doi: 10.1056/NEJM198102193040804. [DOI] [PubMed] [Google Scholar]
- Herberman R. B. Inhibition of natural cytotoxic rabbit antibody by human IgM: production of nontoxic rabbit serum for use as complement source. J Immunol. 1970 Apr;104(4):805–809. [PubMed] [Google Scholar]
- Kumagai S., Steinberg A. D., Green I. Antibodies to T cells in patients with systemic lupus erythematosus can induce antibody-dependent cell-mediated cytotoxicity against human T cells. J Clin Invest. 1981 Mar;67(3):605–614. doi: 10.1172/JCI110074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Abe T., Toguchi T., Kiyotaki M., Homma M. Studies of anti-lymphocyte antibody of patients with active SLE. I. Cause of loss of suppressor T-lymphocyte function. Scand J Immunol. 1979;10(3):213–221. doi: 10.1111/j.1365-3083.1979.tb01342.x. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Reinherz E. L., Abe T., Homma M., Schlossman S. F. Characteristics of anti-T-cell antibodies in systemic lupus erythematosus: evidence for selective reactivity with normal suppressor cells defined by monoclonal antibodies. Clin Immunol Immunopathol. 1980 Aug;16(4):474–484. doi: 10.1016/0090-1229(80)90189-0. [DOI] [PubMed] [Google Scholar]
- Pussell B. A., Blyth F., Charlesworth J. A. Failure to detect brain reactivity of lymphocytotoxins in cerebral lupus. Clin Exp Immunol. 1982 Jan;47(1):133–137. [PMC free article] [PubMed] [Google Scholar]
- Quin J. W., Charlesworth J. A., Bowman C., Macdonald G. J. Studies of lymphocytotoxins in infectious mononucleosis and systemic lupus erythematosus: evidence for immune complex-mediated cytotoxicity. Clin Exp Immunol. 1980 Mar;39(3):593–598. [PMC free article] [PubMed] [Google Scholar]
- Quin J. W., Charlesworth J. A., Lee C. H., Macdonald G. J. Studies of lymphocytotoxins in infectious mononucleosis: reduced lymphocyte killing in the acute phase. Clin Exp Immunol. 1980 Mar;39(3):588–592. [PMC free article] [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Reeves J. P., Green I. Studies of immune functions of patients with systemic lupus erythematosus. Complement-dependent immunoglobulin M anti-thymus-derived cell antibodies preferentially inactivate suppressor cells. J Clin Invest. 1979 May;63(5):954–965. doi: 10.1172/JCI109396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen J. S., Chused T. M., Leiserson W. M., Reeves J. P., Alling D., Steinberg A. D. Heterogeneity of immunoregulatory T-cell subsets in systemic lupus erythematosus. Correlation with clinical features. Am J Med. 1982 May;72(5):783–790. doi: 10.1016/0002-9343(82)90544-7. [DOI] [PubMed] [Google Scholar]
- TERASAKI P. I., MCCLELLAND J. D. MICRODROPLET ASSAY OF HUMAN SERUM CYTOTOXINS. Nature. 1964 Dec 5;204:998–1000. doi: 10.1038/204998b0. [DOI] [PubMed] [Google Scholar]


