Abstract
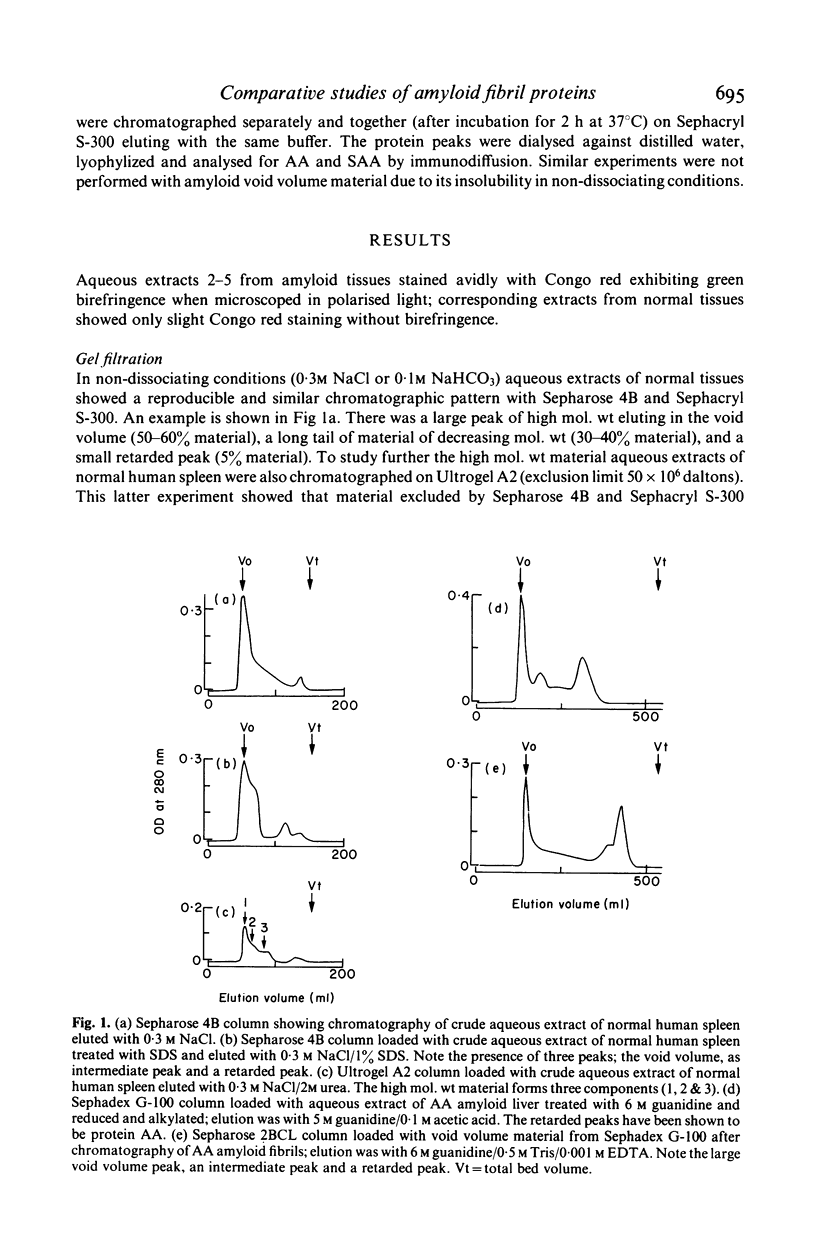
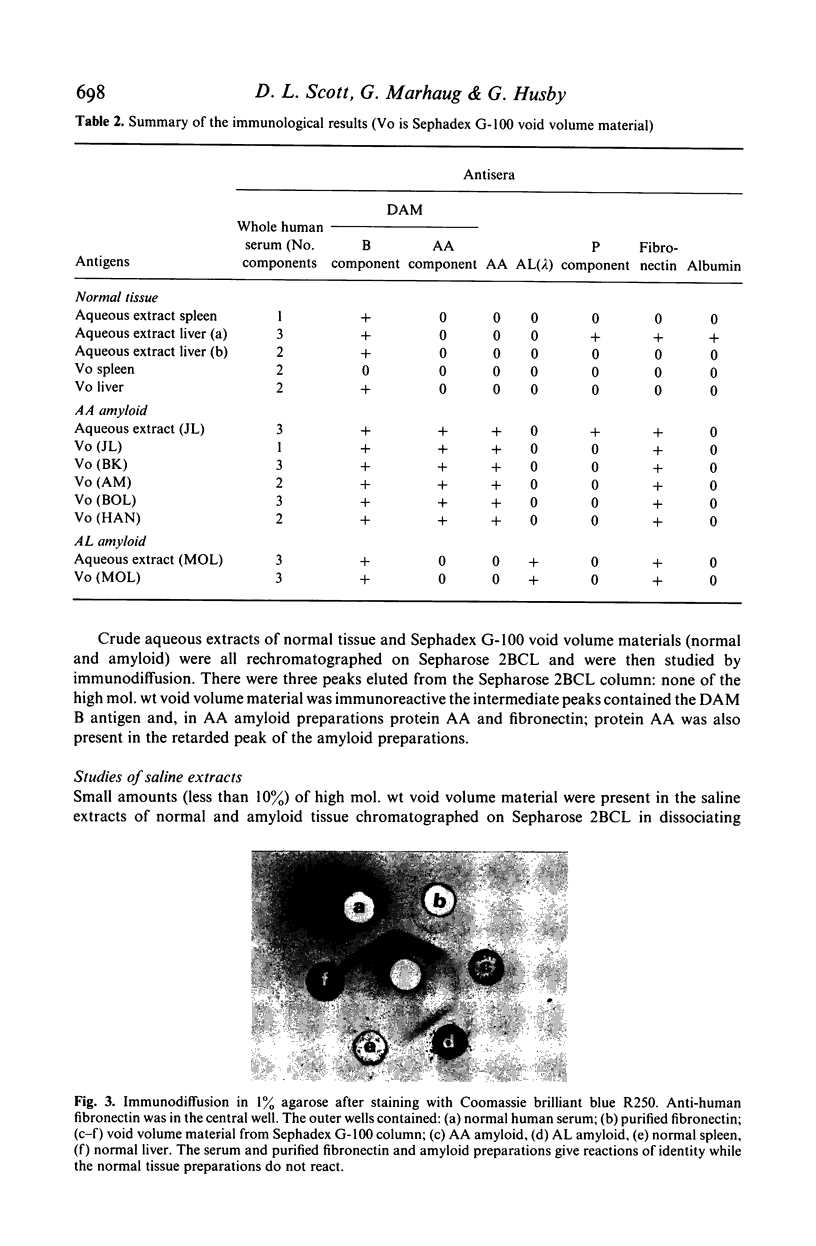
Analysis of purified amyloid fibrils by gel filtration, polyacrylamide gel electrophoresis in SDS and 8 M urea, and immunodiffusion and immunoelectrophoresis showed that, in addition to the specific amyloid proteins AA and AL, the amyloid preparations all contain a high molecular weight complex. The latter protein complex contains fibronectin, a component which reacts with a non-AA specificity of an antiserum to degraded AA amyloid fibrils (termed the 'B' specificity), and a high molecular weight component excluded by a Sepharose 2BCL column. Similar components were found in aqueous extracts of normal tissues prepared by an identical procedure, and these form aggregates of different size in non-dissociating conditions. It is suggested that amyloid fibrils are complexes of a variety of macromolecules in addition to the specific proteins AA and AL.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benditt E. P., Eriksen N. Chemical similarity among amyloid substances associated with long standing inflammation. Lab Invest. 1972 Jun;26(6):615–625. [PubMed] [Google Scholar]
- Costa P. P., Figueira A. S., Bravo F. R. Amyloid fibril protein related to prealbumin in familial amyloidotic polyneuropathy. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4499–4503. doi: 10.1073/pnas.75.9.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner G. G. Amyloid deposits and amyloidosis. The beta-fibrilloses (first of two parts). N Engl J Med. 1980 Jun 5;302(23):1283–1292. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Harada M., Isersky C. The purification of amyloid fibril proteins. Prep Biochem. 1972;2(1):39–51. doi: 10.1080/00327487208061451. [DOI] [PubMed] [Google Scholar]
- Holck M., Husby G., Sletten K., Natvig J. B. The amyloid P-component (protein AP): an integral part of the amyloid substance? Scand J Immunol. 1979;10(1):55–60. doi: 10.1111/j.1365-3083.1979.tb01334.x. [DOI] [PubMed] [Google Scholar]
- Husby G. A chemical classification of amyloid. Correlation with different clinical types of amyloidosis. Scand J Rheumatol. 1980;9(1):60–64. doi: 10.1080/03009748009098131. [DOI] [PubMed] [Google Scholar]
- Husby G., Natvig J. B. Individual antigenic specificity and cross-reactions among amyloid preparations from different individuals. Clin Exp Immunol. 1972 Apr;10(4):635–647. [PMC free article] [PubMed] [Google Scholar]
- Husby G., Natvig J. B., Sletten K. New, third class of amyloid fibril protein. J Exp Med. 1974 Mar 1;139(3):773–778. doi: 10.1084/jem.139.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husby G., Sletten K., Michaelsen T. E., Natvig J. B. Antigenic and chemical characterization of non-immunoglobulin amyloid proteins. Scand J Immunol. 1972;1(4):393–400. doi: 10.1111/j.1365-3083.1972.tb03305.x. [DOI] [PubMed] [Google Scholar]
- Husby G., Sletten K. Structural similarities between a protein extracted from normal human tissues and a component of amyloid fibrils. Acta Pathol Microbiol Scand C. 1977 Jun;85(3):153–160. doi: 10.1111/j.1699-0463.1977.tb03625.x. [DOI] [PubMed] [Google Scholar]
- Levin M., Franklin E. C., Frangione B., Pras M. The amino acid sequence of a major nonimmunoglobulin component of some amyloid fibrils. J Clin Invest. 1972 Oct;51(10):2773–2776. doi: 10.1172/JCI107098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhaug G., Husby G. Characterization of human amyloid-related protein SAA as a polymorphic protein: association with albumin and prealbumin in serum. Clin Exp Immunol. 1981 Jul;45(1):97–106. [PMC free article] [PubMed] [Google Scholar]
- Pras M., Glynn L. E. Isolation of a non-collagenous reticulin component and its primary characterization. Br J Exp Pathol. 1973 Aug;54(4):449–456. [PMC free article] [PubMed] [Google Scholar]
- Pras M., Johnson G. D., Holborow E. J., Glynn L. E. Antigenic properties of a non-collagenous reticulin component of normal connective tissue. Immunology. 1974 Sep;27(3):469–478. [PMC free article] [PubMed] [Google Scholar]
- Pras M., Schubert M., Zucker-Franklin D., Rimon A., Franklin E. C. The characterization of soluble amyloid prepared in water. J Clin Invest. 1968 Apr;47(4):924–933. doi: 10.1172/JCI105784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pras M., Zucker-Franklin D., Rimon A., Franklin E. C. Physical, chemical, and ultrastructural studies of water-soluble human amyloid fibrils. Comparative analyses of nine amyloid preparations. J Exp Med. 1969 Oct 1;130(4):777–796. doi: 10.1084/jem.130.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Hayman E. G., Engvall E., Cothran W. C., Butler W. T. Alignment of biologically active domains in the fibronectin molecule. J Biol Chem. 1981 Jul 25;256(14):7277–7281. [PubMed] [Google Scholar]
- Scott D. L., Bedford P. A., Walton K. W. The preparation of plasma fibronectin antigen and antiserum. J Immunol Methods. 1981;43(1):29–33. doi: 10.1016/0022-1759(81)90033-8. [DOI] [PubMed] [Google Scholar]
- Sletten K., Westermark P., Natvig J. B. Characterization of amyloid fibril proteins from medullary carcinoma of the thyroid. J Exp Med. 1976 Apr 1;143(4):993–998. doi: 10.1084/jem.143.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman S., Vaheri A. Distribution of a major connective tissue protein, fibronectin, in normal human tissues. J Exp Med. 1978 Apr 1;147(4):1054–1064. doi: 10.1084/jem.147.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth D. J., Scott D. L., Almond T. J., Beard H. K., Holborow E. J., Walton K. W. Studies on reticulin. I: Serological and immunohistological investigations of the occurrence of collagen type III, fibronectin and the non-collagenous glycoprotein of Pras and Glynn in reticulin. Br J Exp Pathol. 1982 Apr;63(2):154–166. [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yamada K. M., Kennedy D. W. Fibroblast cellular and plasma fibronectins are similar but not identical. J Cell Biol. 1979 Feb;80(2):492–498. doi: 10.1083/jcb.80.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Olden K. Fibronectins--adhesive glycoproteins of cell surface and blood. Nature. 1978 Sep 21;275(5677):179–184. doi: 10.1038/275179a0. [DOI] [PubMed] [Google Scholar]
- de Beer F. C., Baltz M. L., Holford S., Feinstein A., Pepys M. B. Fibronectin and C4-binding protein are selectively bound by aggregated amyloid P component. J Exp Med. 1981 Oct 1;154(4):1134–1139. doi: 10.1084/jem.154.4.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]