Abstract
The frequency of cells reactive with anti-Tac monoclonal antibody (MoAb), which recognizes the interleukin-2 (IL-2) receptor, has been evaluated in cell suspensions from peripheral blood and bronchoalveolar lavage (BAL), and in frozen sections from involved tissues in 18 patients with active sarcoidosis. Peripheral blood lymphocytes of sarcoid patients do not bear Tac determinant and reduced numbers of Tac+ cells are inducible following PHA stimulation. On the other hand, significant numbers of lymphocytes reactive with anti-TacMoAb are present in the cells obtained from the BAL and a number of Tac+ cells infiltrate the lung, lymph node and conjunctiva. The finding of Tac+ cells in the BAL fluid and in other organs in patients with sarcoidosis provides evidence that some T cells in these involved tissues have the characteristics of IL-2 responder cells and thus the potential to absorb IL-2, supporting the hypothesis that T lymphocytes replicate in situ at sites of disease activity.
Full text
PDF

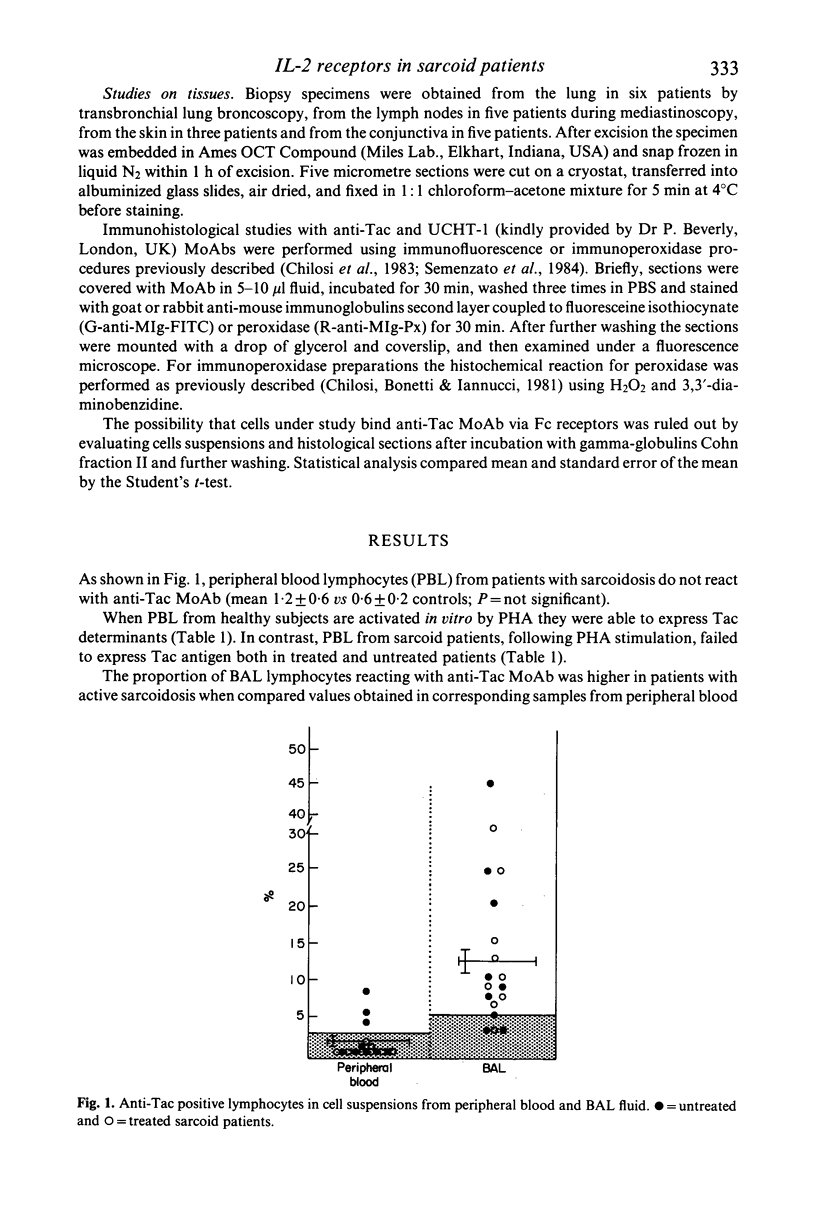



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cantrell D. A., Smith K. A. Transient expression of interleukin 2 receptors. Consequences for T cell growth. J Exp Med. 1983 Dec 1;158(6):1895–1911. doi: 10.1084/jem.158.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilosi M., Pizzolo G., Fiore-Donati L., Bofill M., Janossy G. Routine immunofluorescent and histochemical analysis of bone marrow involvement of lymphoma/leukaemia: the use of cryostat sections. Br J Cancer. 1983 Dec;48(6):763–775. doi: 10.1038/bjc.1983.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo R. C., Kalyanaraman V. S., Sarngadharan M. G., Sliski A., Vonderheid E. C., Maeda M., Nakao Y., Yamada K., Ito Y., Gutensohn N. Association of the human type C retrovirus with a subset of adult T-cell cancers. Cancer Res. 1983 Aug;43(8):3892–3899. [PubMed] [Google Scholar]
- Gallo R. C., Wong-Staal F. Retroviruses as etiologic agents of some animal and human leukemias and lymphomas and as tools for elucidating the molecular mechanism of leukemogenesis. Blood. 1982 Sep;60(3):545–557. [PubMed] [Google Scholar]
- Gillis S., Crabtree G. R., Smith K. A. Glucocorticoid-induced inhibition of T cell growth factor production. I. The effect on mitogen-induced lymphocyte proliferation. J Immunol. 1979 Oct;123(4):1624–1631. [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A., Melchers F. A plaque assay for all cells secreting Ig of a given type or class. Eur J Immunol. 1976 Aug;6(8):588–590. doi: 10.1002/eji.1830060812. [DOI] [PubMed] [Google Scholar]
- Hardt C., Röllinghoff M., Pfizenmaier K., Mosmann H., Wagner H. Lyt-23+ cyclophosphamide-sensitive T cells regulate the activity of an interleukin 2 inhibitor in vivo. J Exp Med. 1981 Aug 1;154(2):262–274. doi: 10.1084/jem.154.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T., Uchiyama T., Toibana T., Takatsuki K., Uchino H. Surface phenotype of Japanese adult T-cell leukemia cells characterized by monoclonal antibodies. Blood. 1981 Sep;58(3):645–647. [PubMed] [Google Scholar]
- Hunninghake G. W., Bedell G. N., Zavala D. C., Monick M., Brady M. Role of interleukin-2 release by lung T-cells in active pulmonary sarcoidosis. Am Rev Respir Dis. 1983 Oct;128(4):634–638. doi: 10.1164/arrd.1983.128.4.634. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Crystal R. G. Pulmonary sarcoidosis: a disorder mediated by excess helper T-lymphocyte activity at sites of disease activity. N Engl J Med. 1981 Aug 20;305(8):429–434. doi: 10.1056/NEJM198108203050804. [DOI] [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Uchiyama T., Smith K. A., Waldmann T. A., Greene W. C. A monoclonal antibody that appears to recognize the receptor for human T-cell growth factor; partial characterization of the receptor. Nature. 1982 Nov 18;300(5889):267–269. doi: 10.1038/300267a0. [DOI] [PubMed] [Google Scholar]
- Ling N. R., Bishop S., Jefferis Use of antibody-coated red cells for the sensitive detection of antigen and in rosette tests for cells bearing surface immunoglobulins. J Immunol Methods. 1977;15(3):279–289. doi: 10.1016/0022-1759(77)90065-5. [DOI] [PubMed] [Google Scholar]
- Modlin R. L., Hofman F. M., Meyer P. R., Sharma O. P., Taylor C. R., Rea T. H. In situ demonstration of T lymphocyte subsets in granulomatous inflammation: leprosy, rhinoscleroma and sarcoidosis. Clin Exp Immunol. 1983 Mar;51(3):430–438. [PMC free article] [PubMed] [Google Scholar]
- Pacheco Y., Cordier G., Perrin-Fayolle M., Revillard J. P. Flow cytometry analysis of T lymphocytes in sarcoidosis. Am J Med. 1982 Jul;73(1):82–88. doi: 10.1016/0002-9343(82)90930-5. [DOI] [PubMed] [Google Scholar]
- Pinkston P., Bitterman P. B., Crystal R. G. Spontaneous release of interleukin-2 by lung T lymphocytes in active pulmonary sarcoidosis. N Engl J Med. 1983 Apr 7;308(14):793–800. doi: 10.1056/NEJM198304073081401. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Greene W. C. Direct demonstration of the identity of T cell growth factor binding protein and the Tac antigen. J Exp Med. 1983 Oct 1;158(4):1332–1337. doi: 10.1084/jem.158.4.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Munck A., Smith K. A. T cell growth factor receptors. Quantitation, specificity, and biological relevance. J Exp Med. 1981 Nov 1;154(5):1455–1474. doi: 10.1084/jem.154.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenzato G., Pezzutto A., Agostini C., Gasparotto G., Cipriani A. Immunoregulation in sarcoidosis. Clin Immunol Immunopathol. 1981 Jun;19(3):416–427. doi: 10.1016/0090-1229(81)90084-2. [DOI] [PubMed] [Google Scholar]
- Semenzato G., Pezzutto A., Chilosi M., Pizzolo G. Redistribution of T lymphocytes in the lymph nodes of patients with sarcoidosis. N Engl J Med. 1982 Jan 7;306(1):48–49. doi: 10.1056/NEJM198201073060114. [DOI] [PubMed] [Google Scholar]
- Semenzato G., Pezzutto A., Pizzolo G., Chilosi M., Ossi E., Angi M. R., Cipriani A. Immunohistological study in sarcoidosis: evaluation at different sites of disease activity. Clin Immunol Immunopathol. 1984 Jan;30(1):29–40. doi: 10.1016/0090-1229(84)90004-7. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Gillis S., Baker P. E., McKenzie D., Ruscetti F. W. T-cell growth factor-mediated T-cell proliferation. Ann N Y Acad Sci. 1979;332:423–432. doi: 10.1111/j.1749-6632.1979.tb47136.x. [DOI] [PubMed] [Google Scholar]
- Taniguchi N., Miyawaki T., Yachie A., Ikuta N., Ohzeki S. Kinetics of expression of T-cell "activation" antigens on in vivo- and in vitro-stimulated T cells. Diagn Immunol. 1983;1(3):104–111. [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Yachie A., Miyawaki T., Uwadana N., Ohzeki S., Taniguchi N. Sequential expression of T cell activation (Tac) antigen and Ia determinants on circulating human T cells after immunization with tetanus toxoid. J Immunol. 1983 Aug;131(2):731–735. [PubMed] [Google Scholar]



