Abstract
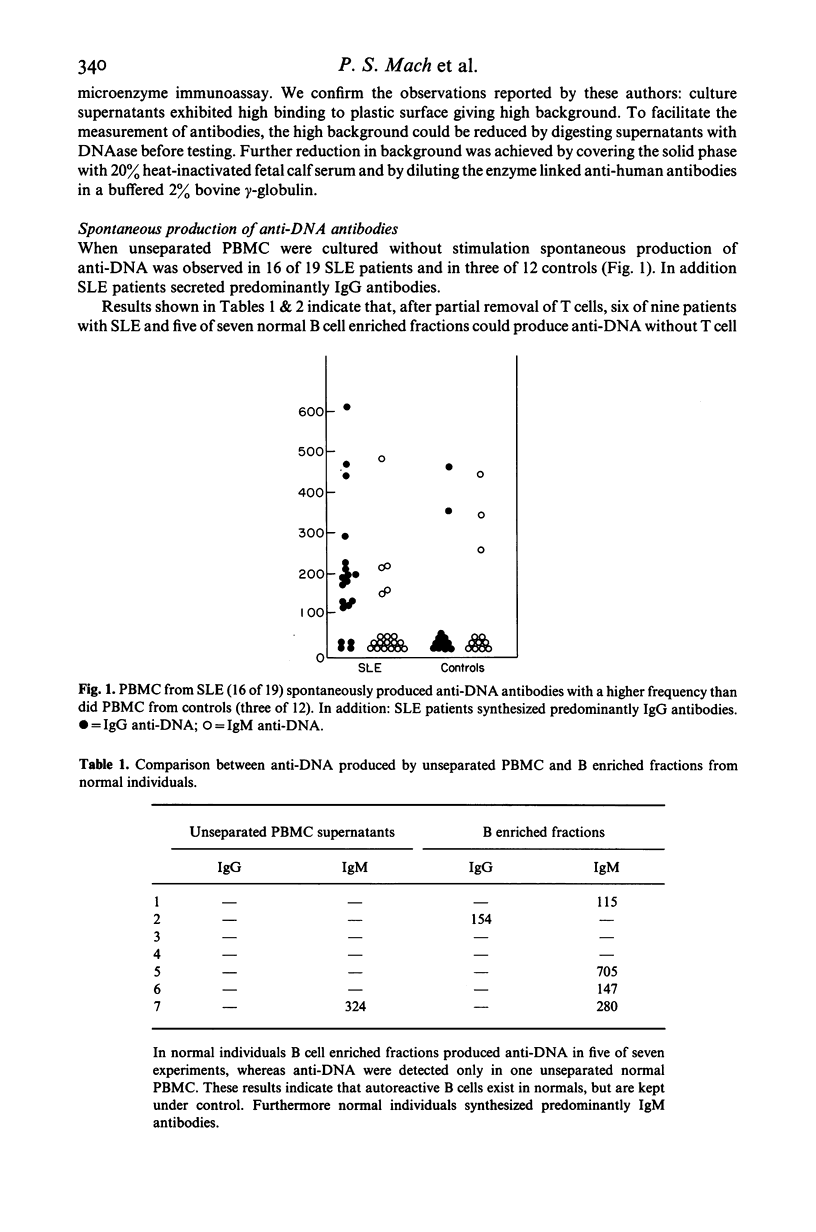
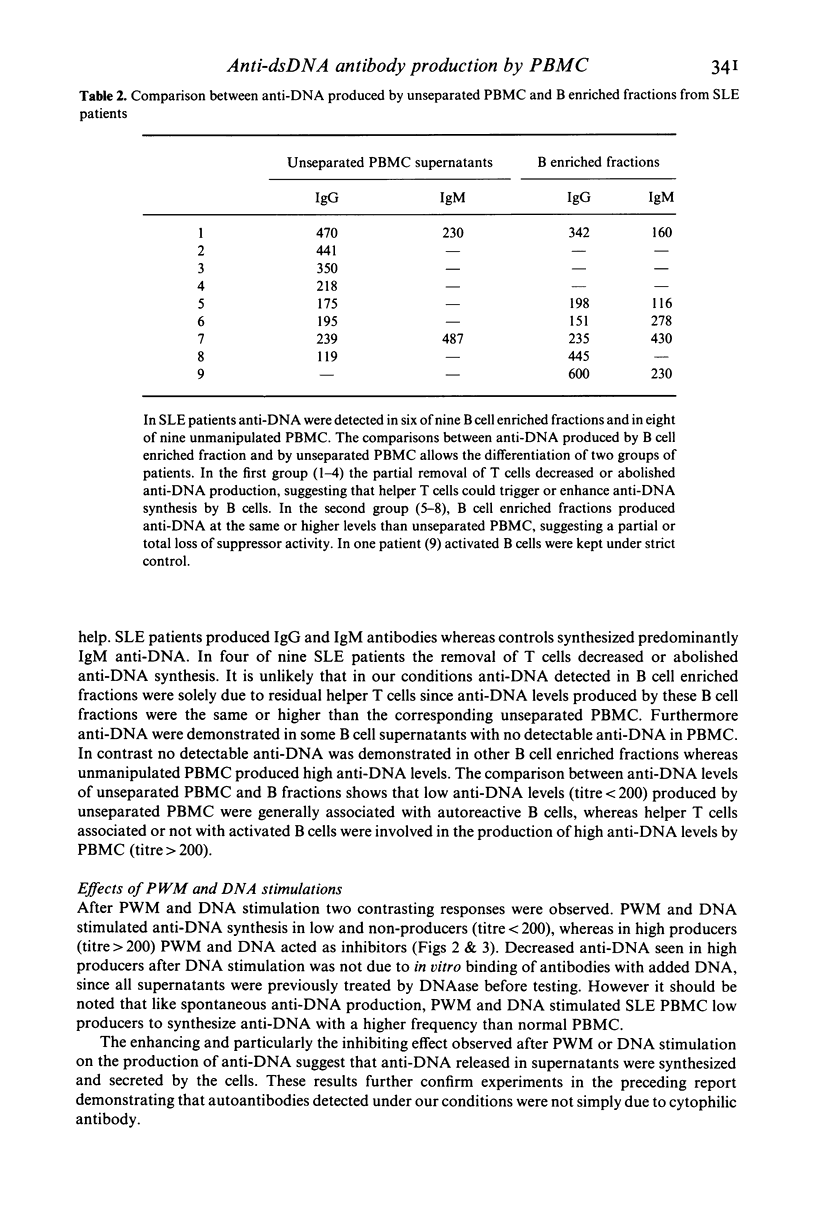
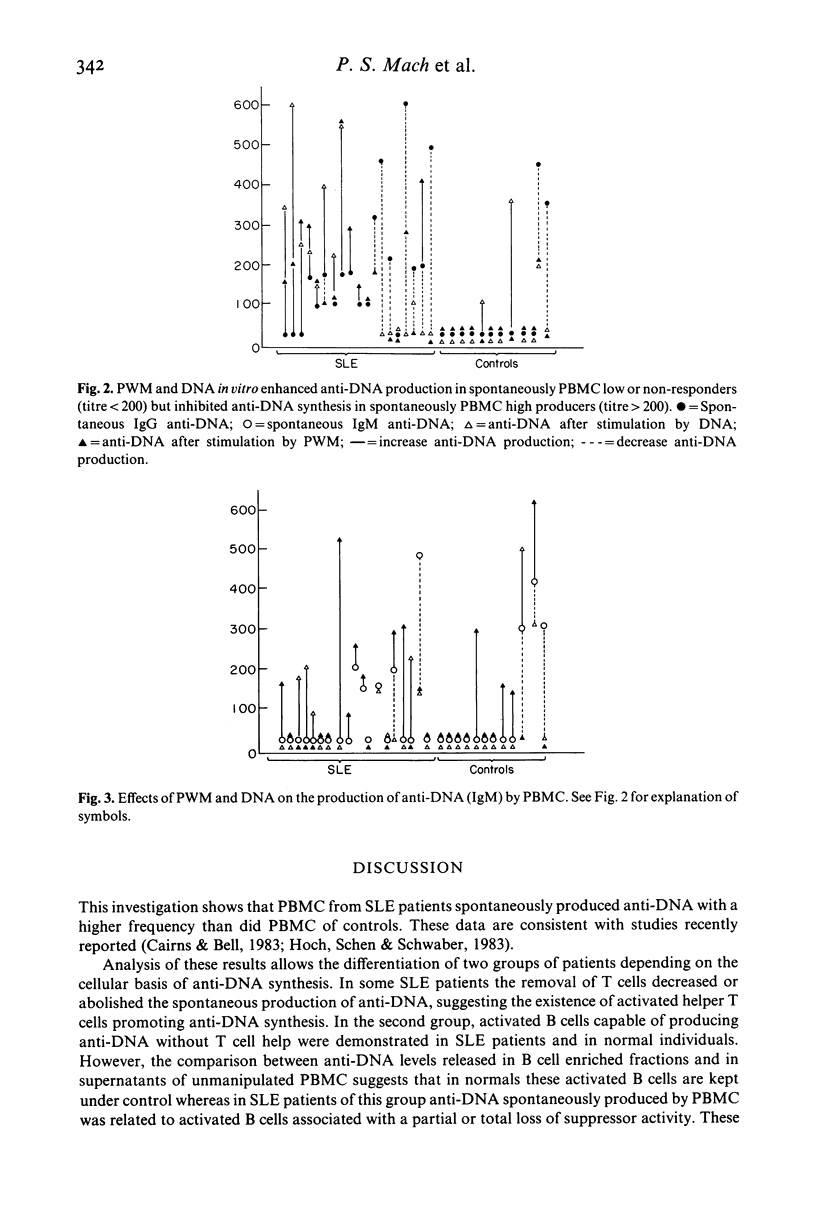
The in vitro production of anti-double stranded DNA antibodies (anti-DNA) by peripheral blood mononuclear cells (PBMC) was investigated in 19 patients with systemic lupus erythematosus (SLE) and in 12 normal individuals, using a micro solid phase enzyme immunoassay. PBMC from SLE patients spontaneously produced anti-DNA with a higher frequency (16 of 19) than did PBMC of controls (three of 12). In addition SLE patients produced predominantly IgG antibodies. PWM and DNA enhanced anti-DNA synthesis is spontaneously low and non-producers, but acted as inhibitors in spontaneously high producers. The partial removal of T cells decreased or abolished anti-DNA synthesis in four of nine SLE patients. In contrast the B cell enriched fractions of five of nine SLE and five of seven normal patients produced the same or higher anti-DNA levels than did the corresponding unseparated PBMC. These results suggest evidence for autoreactive B cells in SLE as well as in normals, and therefore the combination of these autoreactive B cells with helper and/or suppressor T cell disorders could lead to the over production of anti-DNA seen in different patients with SLE.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdou N. I., Sagawa A., Pascual E., Hebert J., Sadeghee S. Suppressor T-cell abnormality in idiopathic systemic lupus erythematosus. Clin Immunol Immunopathol. 1976 Sep;6(2):192–199. doi: 10.1016/0090-1229(76)90110-0. [DOI] [PubMed] [Google Scholar]
- Bresnihan B., Jasin H. E. Suppressor function of peripheral blood mononuclear cells in normal individuals and in patients with systemic lupus erythematosus. J Clin Invest. 1977 Jan;59(1):106–116. doi: 10.1172/JCI108607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budman D. R., Merchant E. B., Steinberg A. D., Doft B., Gershwin M. E., Lizzio E., Reeves J. P. Increased spontaneous activity of antibody-forming cells in the peripheral blood of patients with active SLE. Arthritis Rheum. 1977 Apr;20(3):829–833. doi: 10.1002/art.1780200312. [DOI] [PubMed] [Google Scholar]
- Clough J. D., Frank S. A., Calabrese L. H. Deficiency of T cell mediated regulation of anti-DNA production in systemic lupus erythematosus. Arthritis Rheum. 1980 Jan;23(1):24–29. doi: 10.1002/art.1780230105. [DOI] [PubMed] [Google Scholar]
- Delfraissy J. F., Segond P., Galanaud P., Wallon C., Massias P., Dormont J. Depressed primary in vitro antibody response in untreated systemic lupus erythematosus. T helper cell defect and lack of defective suppressor cell function. J Clin Invest. 1980 Jul;66(1):141–148. doi: 10.1172/JCI109827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S., Steinberg A. D., Haynes B. F., Whalen G. Immunoregulatory aberrations in systemic lupus erythematosus. J Immunol. 1978 Oct;121(4):1473–1479. [PubMed] [Google Scholar]
- Fish F., Ziff M. The in vitro and in vivo induction of anti-double-stranded DNA antibodies in normal and autoimmune mice. J Immunol. 1982 Jan;128(1):409–414. [PubMed] [Google Scholar]
- Ginsburg W. W., Finkelman F. D., Lipsky P. E. Circulating and pokeweed mitogen-induced immunoglobulin-secreting cells in systemic lupus erythematosus. Clin Exp Immunol. 1979 Jan;35(1):76–88. [PMC free article] [PubMed] [Google Scholar]
- Hoch S., Schur P. H., Schwaber J. Frequency of anti-DNA antibody producing cells from normals and patients with systemic lupus erythematosus. Clin Immunol Immunopathol. 1983 Apr;27(1):28–37. doi: 10.1016/0090-1229(83)90053-3. [DOI] [PubMed] [Google Scholar]
- Jasin H. E., Ziff M. Immunoglobulin synthesis by peripheral blood cells in systemic lupus erythematosus. Arthritis Rheum. 1975 May-Jun;18(3):219–228. doi: 10.1002/art.1780180305. [DOI] [PubMed] [Google Scholar]
- Jones B. M. B cell activation by pokeweed mitogen in cultures of normal peripheral blood lymphocytes depleted of T regulator subsets by treatment with OKT4 and OKT8 monoclonal antibodies. Clin Exp Immunol. 1983 Mar;51(3):461–469. [PMC free article] [PubMed] [Google Scholar]
- Keightley R. G., Cooper M. D., Lawton A. R. The T cell dependence of B cell differentiation induced by pokeweed mitogen. J Immunol. 1976 Nov;117(5 Pt 1):1538–1544. [PubMed] [Google Scholar]
- Krakauer R. S., Clough J. D., Alexander T., Sundeen J., Sauder D. N. Suppressor cell defect in SLE: relationship to native DNA binding. Clin Exp Immunol. 1980 Apr;40(1):72–76. [PMC free article] [PubMed] [Google Scholar]
- Messner R. P., Lindström F. D., Williams R. C., Jr Peripheral blood lymphocyte cell surface markers during the course of systemic lupus erythematosus. J Clin Invest. 1973 Dec;52(12):3046–3056. doi: 10.1172/JCI107503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta L., Webb S. R., Grossi C. E., Lydyard P. M., Cooper M. D. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med. 1977 Jul 1;146(1):184–200. doi: 10.1084/jem.146.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton J. I., Siegel B. V. Radiation sensitivity of New Zealand black mice and the development of autoimmune disease and neoplasia. Proc Natl Acad Sci U S A. 1971 Jan;68(1):124–126. doi: 10.1073/pnas.68.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelton B. K., Denman A. M. Defective B cell function in systemic lupus erythematosus. Clin Exp Immunol. 1982 Jun;48(3):513–518. [PMC free article] [PubMed] [Google Scholar]
- Reeves J. P., Taurog J. D., Steinberg A. D. Polyclonal B-cell activation of autoantibodies (CBA/N x NZB)F1 mice by polyinosinic polycytidylic acid. Clin Immunol Immunopathol. 1981 May;19(2):170–180. doi: 10.1016/0090-1229(81)90060-x. [DOI] [PubMed] [Google Scholar]
- Saxon A., Feldhaus J., Robins R. A. Single step separation of human T and B cells using AET treated srbc rosettes. J Immunol Methods. 1976;12(3-4):285–288. doi: 10.1016/0022-1759(76)90050-8. [DOI] [PubMed] [Google Scholar]
- Scher I., Frantz M. M., Steinberg A. D. The genetics of the immune response to a synthetic double-stranded RNA in a mutant CBA mouse strain. J Immunol. 1973 May;110(5):1396–1401. [PubMed] [Google Scholar]
- Smolen J. S., Chused T. M., Leiserson W. M., Reeves J. P., Alling D., Steinberg A. D. Heterogeneity of immunoregulatory T-cell subsets in systemic lupus erythematosus. Correlation with clinical features. Am J Med. 1982 May;72(5):783–790. doi: 10.1016/0002-9343(82)90544-7. [DOI] [PubMed] [Google Scholar]
- Steinberg A. D., Daley G. G., Talal N. Tolerance to polyinosinic-polycytidylic acid in NZB-NZW mice. Science. 1970 Feb 6;167(3919):870–871. doi: 10.1126/science.167.3919.870. [DOI] [PubMed] [Google Scholar]
- Tan P., Pang G., Wilson J. D. Immunoglobulin production in vitro by peripheral blood lymphocytes in systemic lupus erythematosus: helper T cell defect and B cell hyperreactivity. Clin Exp Immunol. 1981 Jun;44(3):548–554. [PMC free article] [PubMed] [Google Scholar]


