Abstract
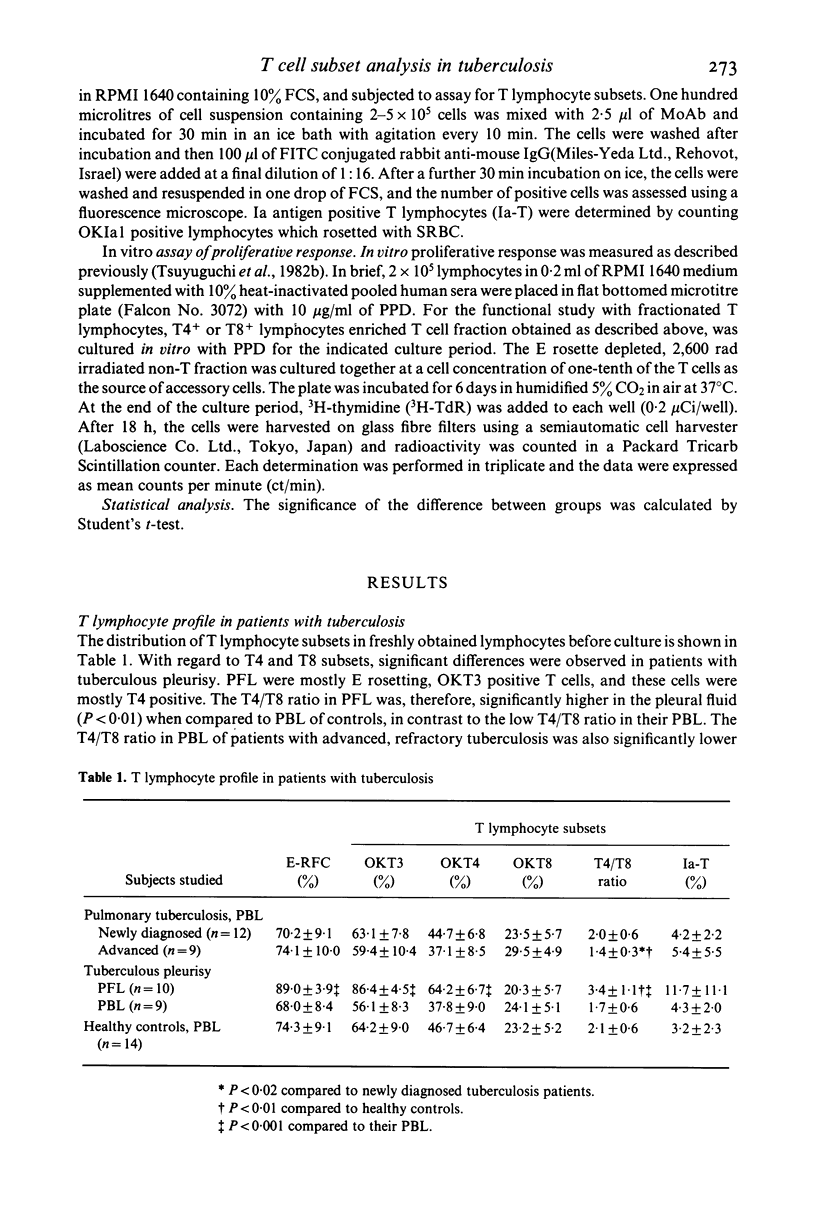
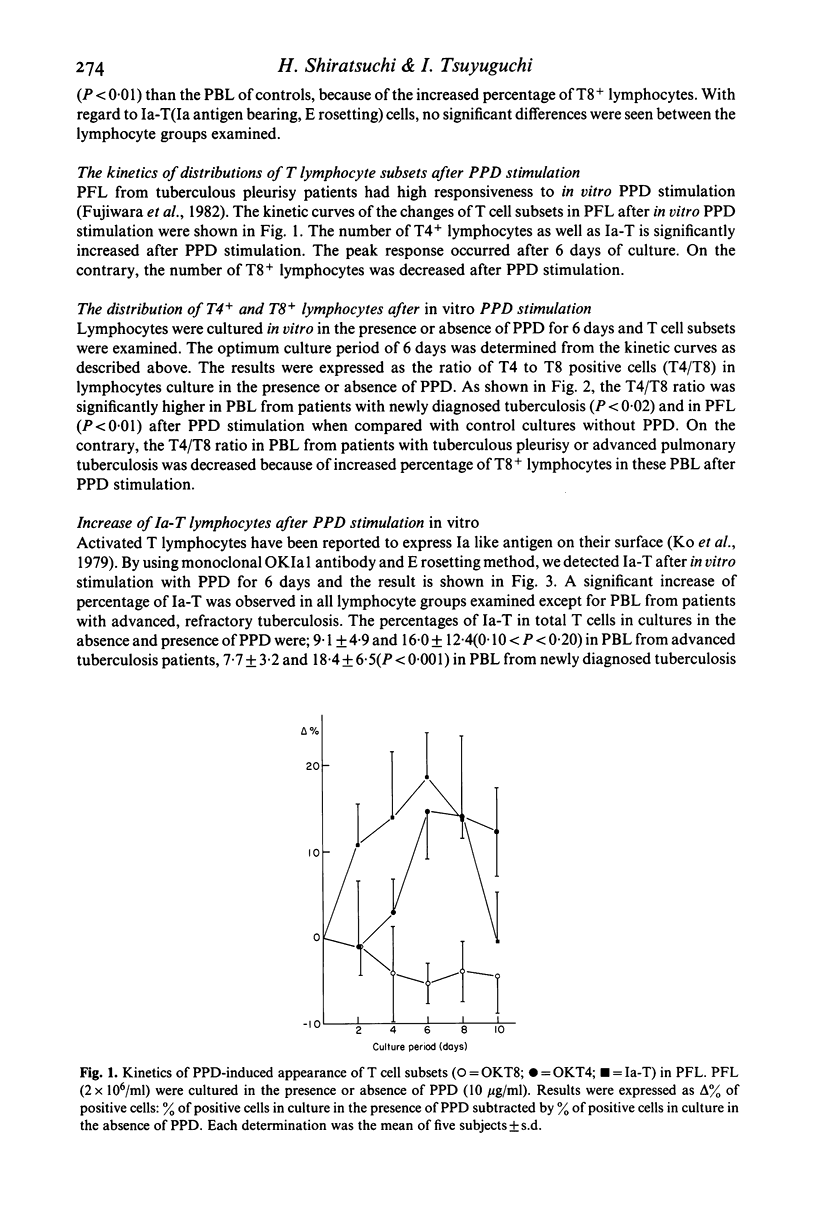
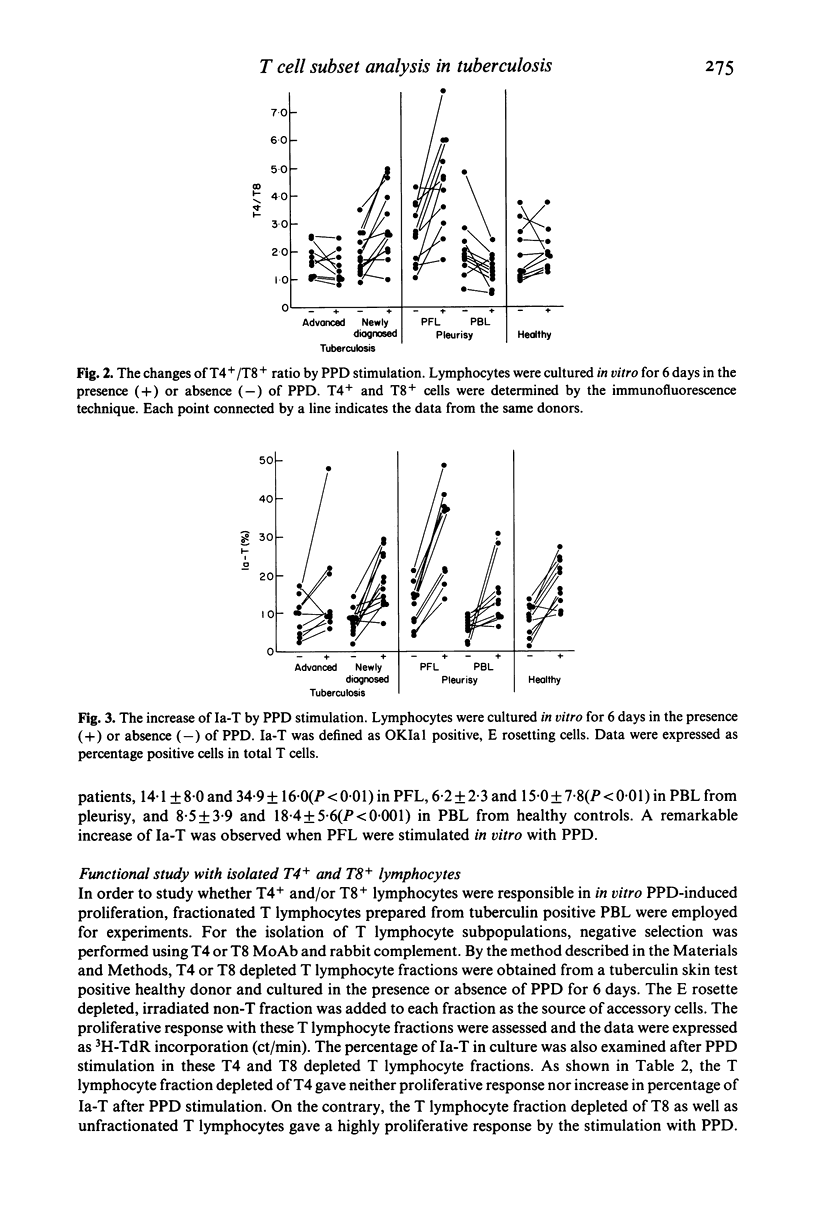
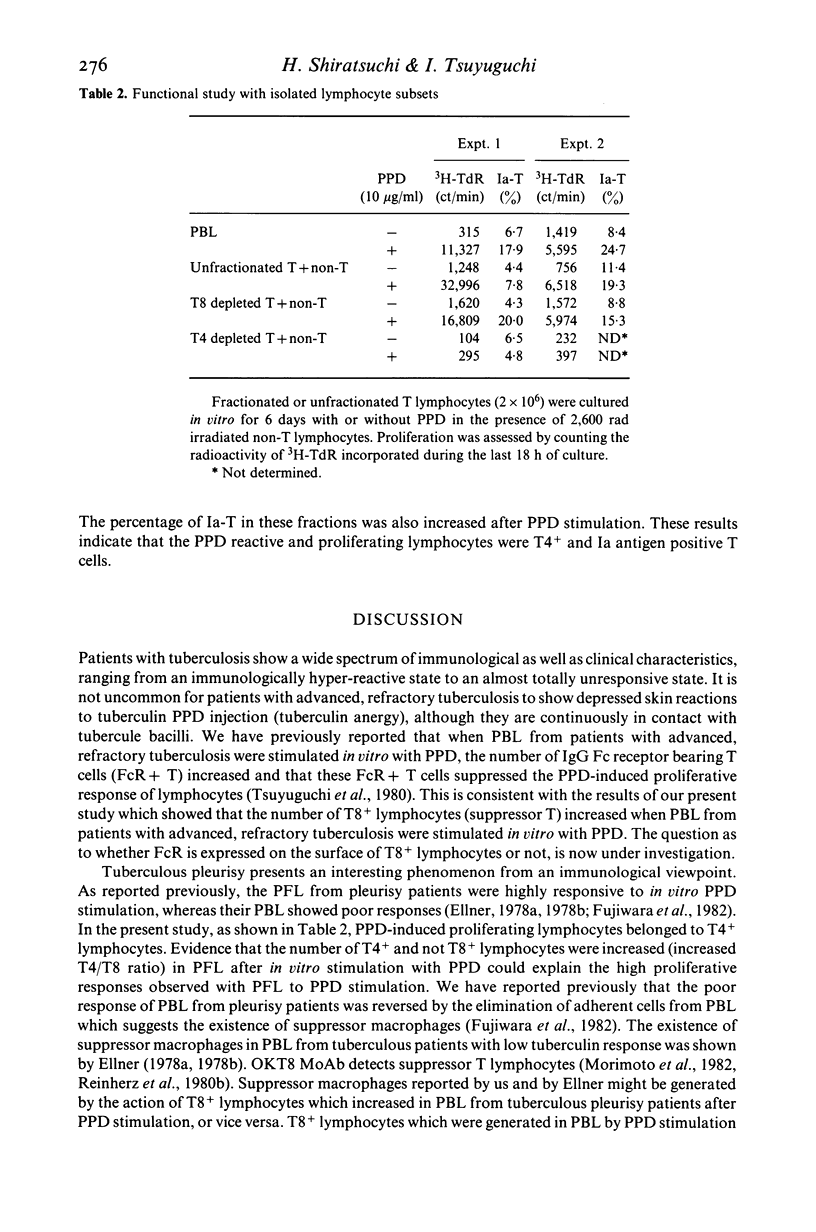
By using OKT monoclonal antibodies; OKT3(pan T), OKT4(inducer/helper), OKT8 (suppressor/cytotoxic) and OKIa1, T lymphocyte subsets were examined in lymphocytes of patients with tuberculosis both before and after in vitro stimulation with purified protein derivative of tuberculin (PPD). In freshly obtained lymphocytes samples before culture, a significantly high T4/T8 ratio in pleural fluid lymphocytes (PFL) from patients with tuberculous pleurisy was observed as compared with either their PBL, or the PBL from healthy controls. In addition, PFL from patients with tuberculous pleurisy showed increased numbers of E rosetting (E-RFC), OKT3+ and OKT4+ cells as compared with their PBL. A low T4/T8 ratio was also observed in PBL of patients with advanced, refractory tuberculosis. After stimulation with PPD in vitro, the T4/T8 ratio increased further in PFL as well as in PBL from patients with newly diagnosed, fresh tuberculosis. Investigation of fractionated T lymphocyte subsets revealed that PPD-induced proliferating lymphocytes belonged to T4+ and not T8+ lymphocytes. Ia antigen bearing T lymphocytes (Ia-T) were increased in all lymphocyte groups studied after in vitro stimulation with PPD. In particular, a remarkable increase was observed when PFL were stimulated in vitro with PPD. Our results suggest that the clinical features of tuberculosis reflect the immunological activity of T lymphocyte subsets in this disease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach M. A., Bach J. F. The use of monoclonal anti-T cell antibodies to study T cell imbalances in human diseases. Clin Exp Immunol. 1981 Sep;45(3):449–456. [PMC free article] [PubMed] [Google Scholar]
- Ellner J. J. Pleural fluid and peripheral blood lymphocyte function in tuberculosis. Ann Intern Med. 1978 Dec;89(6):932–933. doi: 10.7326/0003-4819-89-6-932. [DOI] [PubMed] [Google Scholar]
- Ellner J. J. Suppressor adherent cells in human tuberculosis. J Immunol. 1978 Dec;121(6):2573–2579. [PubMed] [Google Scholar]
- Engleman E. G., Benike C. J., Grumet F. C., Evans R. L. Activation of human T lymphocyte subsets: helper and suppressor/cytotoxic T cells recognize and respond to distinct histocompatibility antigens. J Immunol. 1981 Nov;127(5):2124–2129. [PubMed] [Google Scholar]
- Fujiwara H., Okuda Y., Fukukawa T., Tsuyuguchi I. In vitro tuberculin reactivity of lymphocytes from patients with tuberculous pleurisy. Infect Immun. 1982 Feb;35(2):402–409. doi: 10.1128/iai.35.2.402-409.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatenby P. A., Kotzin B. L., Engleman E. G. Induction of immunoglobulin secreting cells in the human autologous mixed leukocyte reaction: regulation by helper and suppressor lymphocyte subsets defined with monoclonal antibodies. J Immunol. 1981 Nov;127(5):2130–2135. [PubMed] [Google Scholar]
- Jackson R. A., Morris M. A., Haynes B. F., Eisenbarth G. S. Increased circulating Ia-antigen-bearing T cells in type I diabetes mellitus. N Engl J Med. 1982 Apr 1;306(13):785–788. doi: 10.1056/NEJM198204013061305. [DOI] [PubMed] [Google Scholar]
- Ko H. S., Fu S. M., Winchester R. J., Yu D. T., Kunkel H. G. Ia determinants on stimulated human T lymphocytes. Occurrence on mitogen- and antigen-activated T cells. J Exp Med. 1979 Aug 1;150(2):246–255. doi: 10.1084/jem.150.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung P., Goldstein G., Reinherz E. L., Schlossman S. F. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979 Oct 19;206(4416):347–349. doi: 10.1126/science.314668. [DOI] [PubMed] [Google Scholar]
- Mascart-Lemone F., Delespesse G., Servais G., Kunstler M. Characterization of immunoregulatory T lymphocytes during ageing by monoclonal antibodies. Clin Exp Immunol. 1982 Apr;48(1):148–154. [PMC free article] [PubMed] [Google Scholar]
- Morimoto C., Distaso J. A., Borel Y., Schlossman S. F., Reinherz E. L. Communicative interactions between subpopulations of human T lymphocytes required for generation of suppressor effector function in a primary antibody response. J Immunol. 1982 Apr;128(4):1645–1650. [PubMed] [Google Scholar]
- Nagel J. E., Chrest F. J., Adler W. H. Enumeration of T lymphocyte subsets by monoclonal antibodies in young and aged humans. J Immunol. 1981 Nov;127(5):2086–2088. [PubMed] [Google Scholar]
- Phan-Dinh-Tuy F., Durandy A., Griscelli C., Bach M. A. T-cell subset analysis by monoclonal antibodies in primary immunodeficiencies. Scand J Immunol. 1981 Aug;14(2):193–200. doi: 10.1111/j.1365-3083.1981.tb00199.x. [DOI] [PubMed] [Google Scholar]
- Raeman F., De Cock W., De Beukelaar T., De Cree J., Verhaegen H. Enumeration of T lymphocytes subsets in autoimmune disease using monoclonal antibodies. Clin Exp Immunol. 1981 Sep;45(3):475–479. [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Breard J. M., Goldstein G., Schlossman S. F. T cell requirements for generation of helper factor(s) in man: analysis of the subsets involved. J Immunol. 1980 Apr;124(4):1883–1887. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Further characterization of the human inducer T cell subset defined by monoclonal antibody. J Immunol. 1979 Dec;123(6):2894–2896. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4061–4065. doi: 10.1073/pnas.76.8.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Pesando J. M., Ritz J., Goldstein G., Schlossman S. F. Ia determinants on human T-cell subsets defined by monoclonal antibody. Activation stimuli required for expression. J Exp Med. 1979 Dec 1;150(6):1472–1482. doi: 10.1084/jem.150.6.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Morimoto C., Penta A. C., Schlossman S. F. Regulation of B cell immunoglobulin secretion by functional subsets of T lymphocytes in man. Eur J Immunol. 1980 Jul;10(7):570–572. doi: 10.1002/eji.1830100715. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Morimoto C., Penta A. C., Schlossman S. F. Subpopulations of the T4+ inducer T cell subset in man: evidence for an amplifier population preferentially expressing Ia antigen upon activation. J Immunol. 1981 Jan;126(1):67–70. [PubMed] [Google Scholar]
- Tsuyuguchi I., Shiratsuchi H., Fujiwara H. Increase in rosette-forming T cells with autologous human erythrocytes in lymphocytes of patients with tuberculosis by in vitro stimulation with purified protein derivative. Int Arch Allergy Appl Immunol. 1982;67(2):161–168. doi: 10.1159/000233008. [DOI] [PubMed] [Google Scholar]
- Tsuyuguchi I., Shiratsuchi H., Fujiwara H., Teraoka O. Nonspecific recruitment of lymphocytes in purified protein derivative-induced lymphocyte proliferative response of patients with tuberculosis. Infect Immun. 1982 Aug;37(2):702–709. doi: 10.1128/iai.37.2.702-709.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuyuguchi I., Shiratsuchi H., Teraoka O., Hirano T. Increase in T cells bearing IgG Fc receptors in peripheral blood of patients with tuberculosis by in vitro stimulation with purified protein derivative. Am Rev Respir Dis. 1980 Jun;121(6):951–957. doi: 10.1164/arrd.1980.121.6.951. [DOI] [PubMed] [Google Scholar]


