Abstract
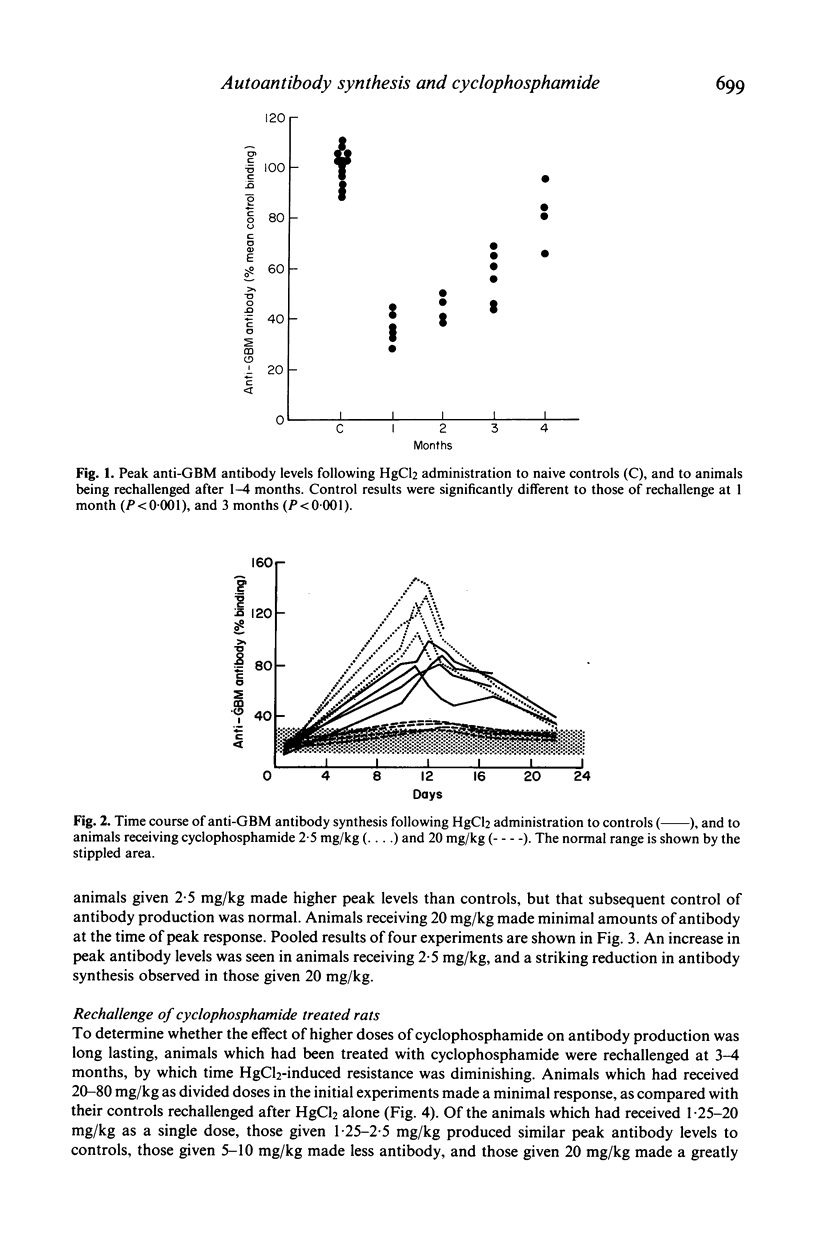
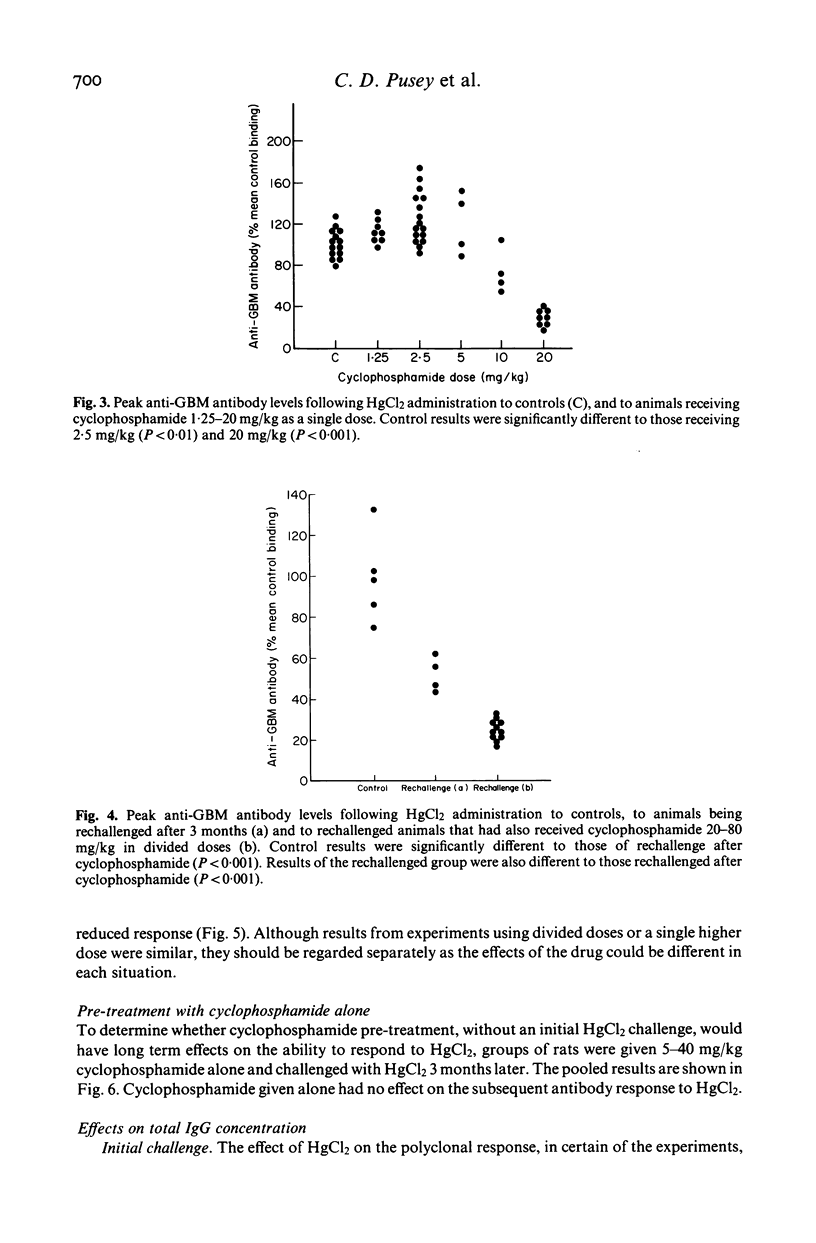
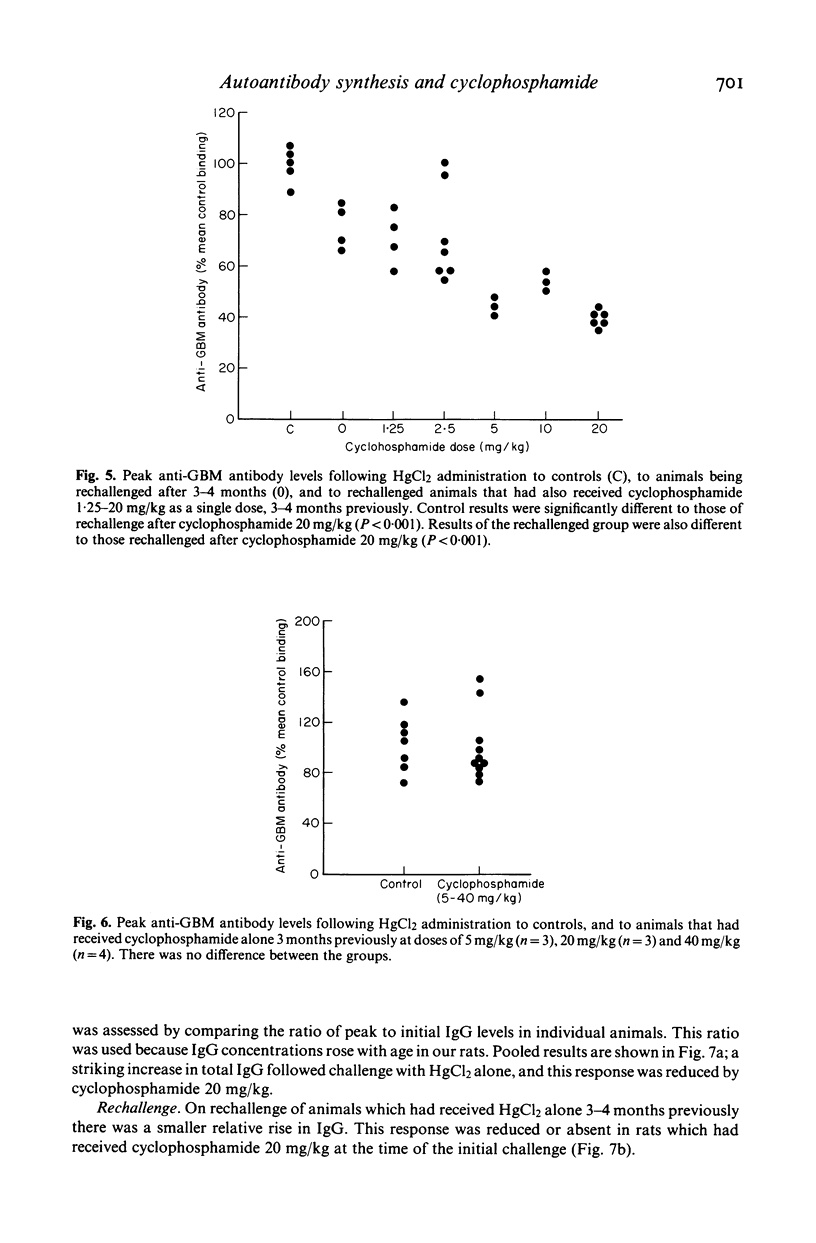
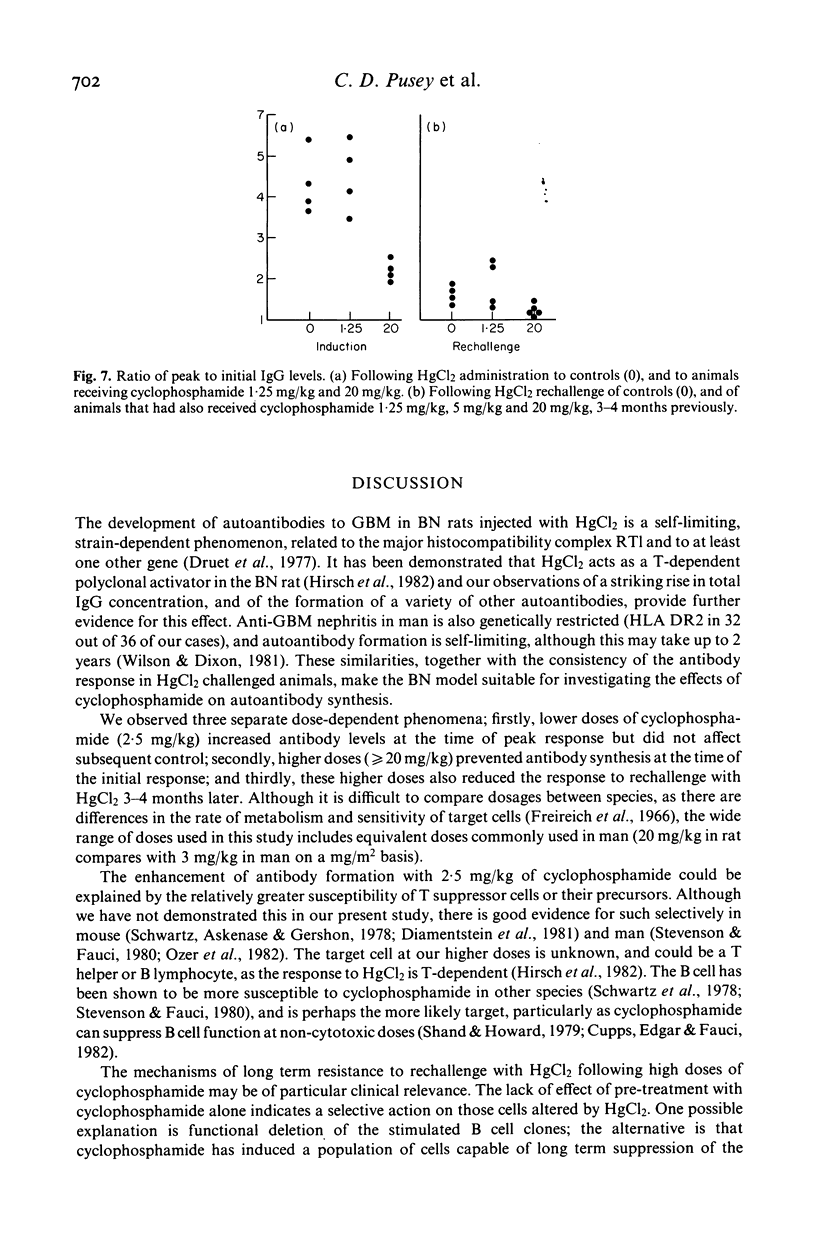
The effects of cyclophosphamide on autoantibody synthesis were studied in an experimental model of glomerulonephritis due to autoantibodies to the glomerular basement membrane (GBM). Brown Norway rats develop anti-GBM antibodies, as part of a polyclonal response, when repeatedly injected with mercuric chloride (HgCl2). Anti-GBM antibody levels peak between days 11 and 14 and thereafter rapidly fall; convalescent animals show a time-dependent resistance to rechallenge with HgCl2 which remains significant for up to 3 months. The administration of cyclophosphamide, as a single intramuscular injection at day 0, has three distinct dose-dependent effects on anti-GBM antibody production. Firstly, lower doses (2.5 mg/kg) increase antibody levels at the time of peak response; secondly, higher doses (greater than or equal to 20 mg/kg) prevent antibody synthesis following HgCl2; and thirdly, the higher doses also reduce the response to rechallenge with HgCl2 3-4 months later. These effects of cyclophosphamide also apply to the polyclonal response to HgCl2, as judged by measurement of total IgG concentrations. Further investigation of the mechanisms of action of cyclophosphamide in this model should provide information relevant to the treatment of human autoimmune disease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appel S. H., Almon R. R., Levy N. Acetylcholine receptor antibodies in myasthenia gravis. N Engl J Med. 1975 Oct 9;293(15):760–761. doi: 10.1056/NEJM197510092931508. [DOI] [PubMed] [Google Scholar]
- Bowman C., Peters D. K., Lockwood C. M. Anti-glomerular basement membrane autoantibodies in the Brown Norway rat: detection by a solid-phase radioimmunoassay. J Immunol Methods. 1983 Jul 29;61(3):325–333. doi: 10.1016/0022-1759(83)90227-2. [DOI] [PubMed] [Google Scholar]
- Cupps T. R., Edgar L. C., Fauci A. S. Suppression of human B lymphocyte function by cyclophosphamide. J Immunol. 1982 Jun;128(6):2453–2457. [PubMed] [Google Scholar]
- Diamantstein T., Klos M., Hahn H., Kaufmann S. H. Direct in vitro evidence for different susceptibilities to 4-hydroperoxycyclophosphamide of antigen-primed T cells regulating humoral and cell-mediated immune responses to sheep erythrocytes: a possible explanation for the inverse action of cyclophosphamide on humoral and cell-mediated immune responses. J Immunol. 1981 May;126(5):1717–1719. [PubMed] [Google Scholar]
- Druet E., Sapin C., Günther E., Feingold N., Druet P. Mercuric chloride-induced anti-glomerular basement membrane antibodies in the rat: genetic control. Eur J Immunol. 1977 Jun;7(6):348–351. doi: 10.1002/eji.1830070605. [DOI] [PubMed] [Google Scholar]
- Freireich E. J., Gehan E. A., Rall D. P., Schmidt L. H., Skipper H. E. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep. 1966 May;50(4):219–244. [PubMed] [Google Scholar]
- Hirsch F., Couderc J., Sapin C., Fournie G., Druet P. Polyclonal effect of HgCl2 in the rat, its possible role in an experimental autoimmune disease. Eur J Immunol. 1982 Jul;12(7):620–625. doi: 10.1002/eji.1830120716. [DOI] [PubMed] [Google Scholar]
- Jones J. V., Robinson M. F., Parciany R. K., Layfer L. F., McLeod B. Therapeutic plasmapheresis in systemic lupus erythematosus. Effect on immune complexes and antibodies to DNA. Arthritis Rheum. 1981 Sep;24(9):1113–1120. doi: 10.1002/art.1780240901. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Glassock R. J., Dixon F. J. The role of anti-glomerular basement membrane antibody in the pathogenesis of human glomerulonephritis. J Exp Med. 1967 Dec 1;126(6):989–1004. doi: 10.1084/jem.126.6.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood C. M., Rees A. J., Pearson T. A., Evans D. J., Peters D. K., Wilson C. B. Immunosuppression and plasma-exchange in the treatment of Goodpasture's syndrome. Lancet. 1976 Apr 3;1(7962):711–715. doi: 10.1016/s0140-6736(76)93089-0. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Ozer H., Cowens J. W., Colvin M., Nussbaum-Blumenson A., Sheedy D. In vitro effects of 4-hydroperoxycyclophosphamide on human immunoregulatory T subset function. I. Selective effects on lymphocyte function in T-B cell collaboration. J Exp Med. 1982 Jan 1;155(1):276–290. doi: 10.1084/jem.155.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters D. K., Rees A. J., Lockwood C. M., Pusey C. D. Treatment and prognosis in antibasement membrane antibody-mediated nephritis. Transplant Proc. 1982 Sep;14(3):513–521. [PubMed] [Google Scholar]
- Sapin C., Druet E., Druet P. Induction of anti-glomerular basement membrane antibodies in the Brown-Norway rat by mercuric chloride. Clin Exp Immunol. 1977 Apr;28(1):173–179. [PMC free article] [PubMed] [Google Scholar]
- Schwartz A., Askenase P. W., Gershon R. K. Regulation of delayed-type hypersensitivity reactions by cyclophosphamide-sensitive T cells. J Immunol. 1978 Oct;121(4):1573–1577. [PubMed] [Google Scholar]
- Shand F. L., Howard J. G. Induction in vitro of reversible immunosuppression and inhibition of B cell receptor regeneration by defined metabolites of cyclophosphamide. Eur J Immunol. 1979 Jan;9(1):17–21. doi: 10.1002/eji.1830090105. [DOI] [PubMed] [Google Scholar]
- Stevenson H. C., Fauci A. S. Activation of human B lymphocytes. XII. Differential effects of in vitro cyclophosphamide on human lymphocyte subpopulations involved in B-cell activation. Immunology. 1980 Mar;39(3):391–397. [PMC free article] [PubMed] [Google Scholar]


