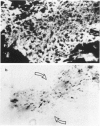
Abstract
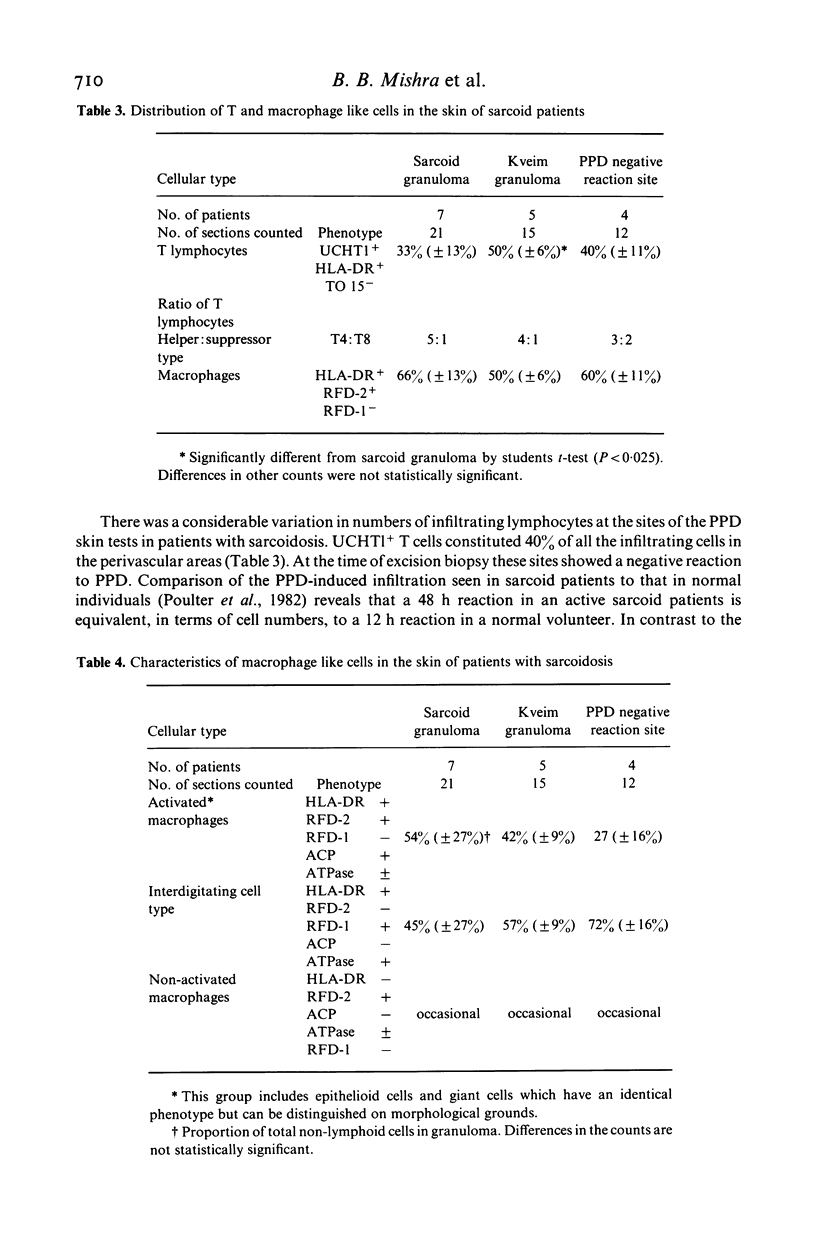
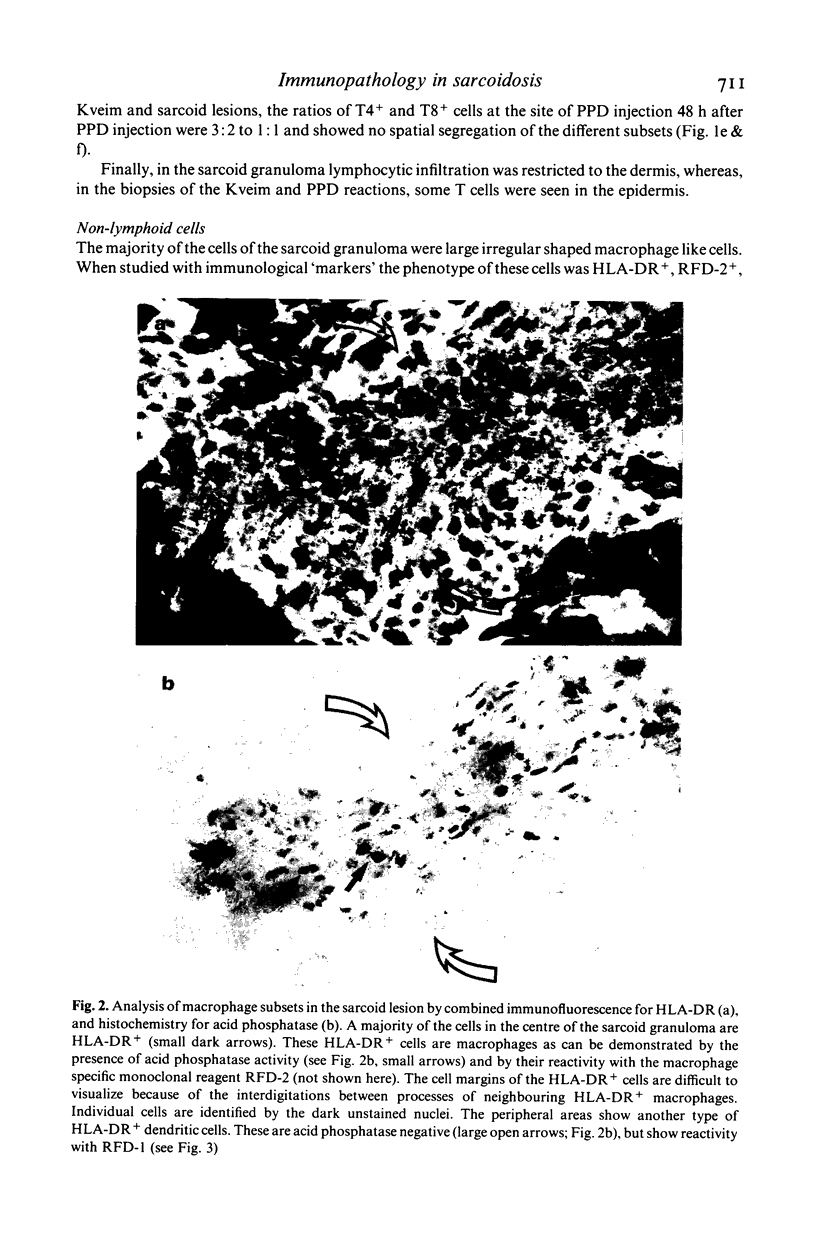
Immunohistological observations of lymphoid and non-lymphoid cell subsets in biopsies of sarcoid skin granulomas have been compared with positive Kveim tests and the sites of PPD injection in sarcoid patients. Monoclonal antibodies have been used in indirect immunofluorescence often in combination with histochemical methods for the detailed characterization of the cells involved. The antibodies used included two new reagents, RFD-1 and RFD-2, which react with interdigitating cells and acid phosphatase positive macrophages, respectively. Sarcoid granulomas had a distinctive pattern of organization though there was a heterogeneity of macrophage like and T lymphoid cells. In the centre, predominantly HLA-DR+, acid phosphatase positive macrophages (RFD-2+) were seen and the lymphoid cells were almost exclusively T4+. At the periphery of the granulomas the HLA-DR+ dendritic cells were ACP negative and RFD-1+. Here T8+ cells were admixed with the T4+ population. The Kveim granuloma had fewer RFD-2+, macrophages and therefore the RFD-1+ cells were more evenly distributed, but the other cells showed a similar distribution to the established lesions. The PPD injection sites contained fewer T cells than the normal control infiltrates in PPD positive healthy individuals. The T4+/T8+ ratios were about 3:2. The most likely explanation for the PPD anergy in sarcoidosis is the sluggish traffic of T4+ cells which could be due to the sequestration of T4+ cells in sites of ongoing inflammation.
Full text
PDF

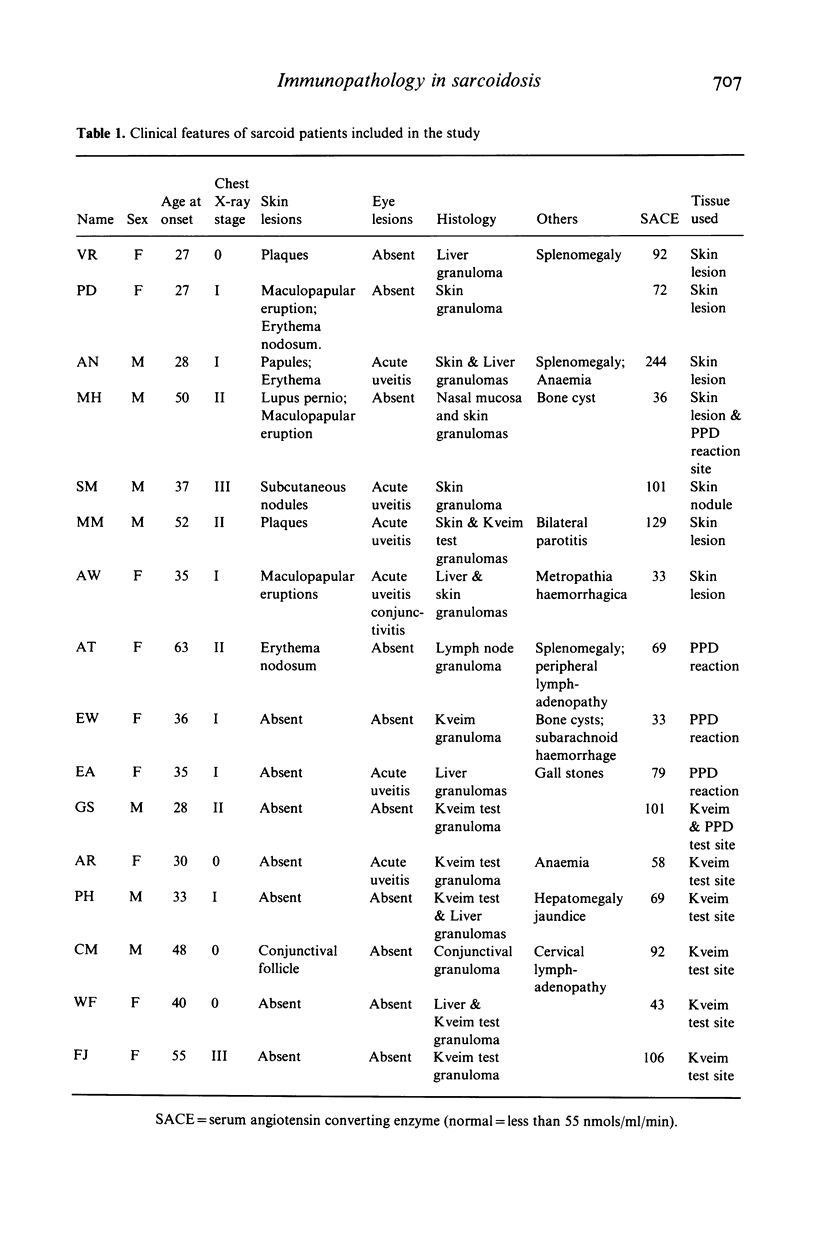
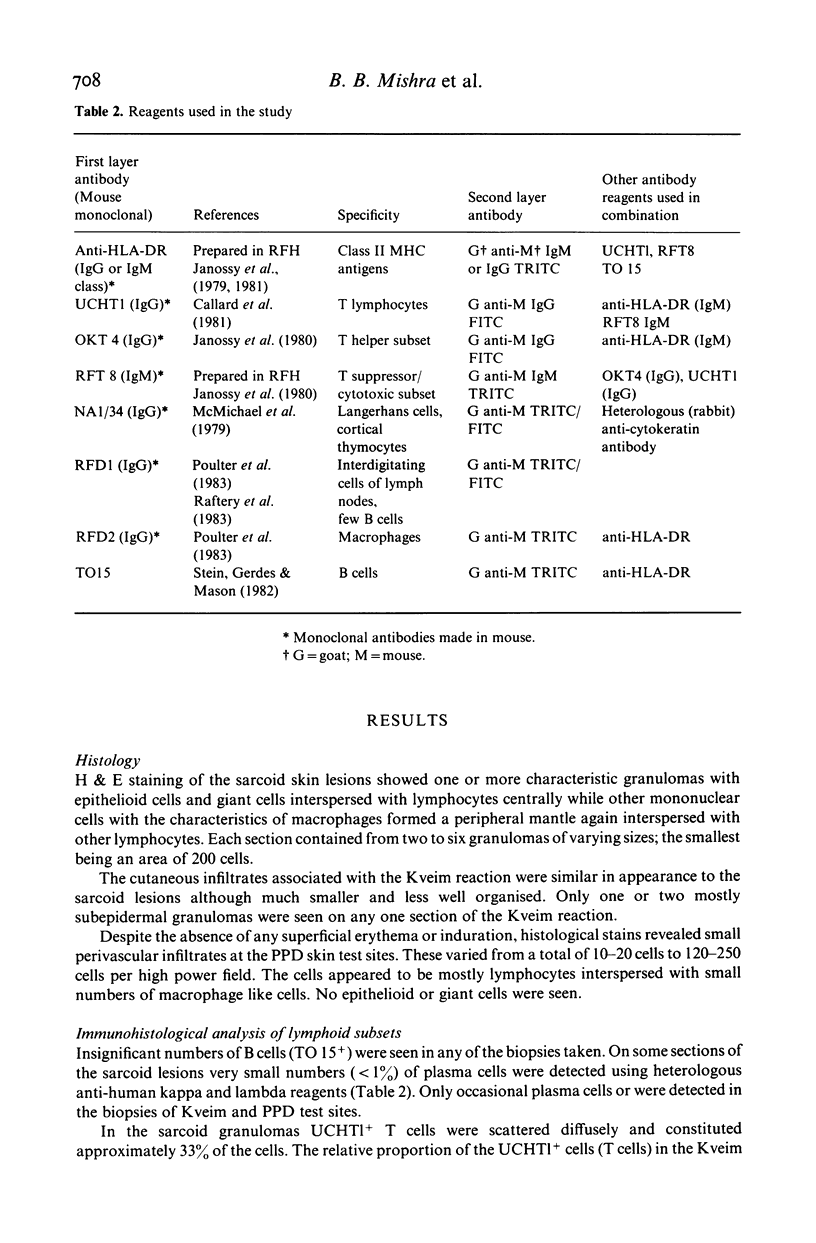
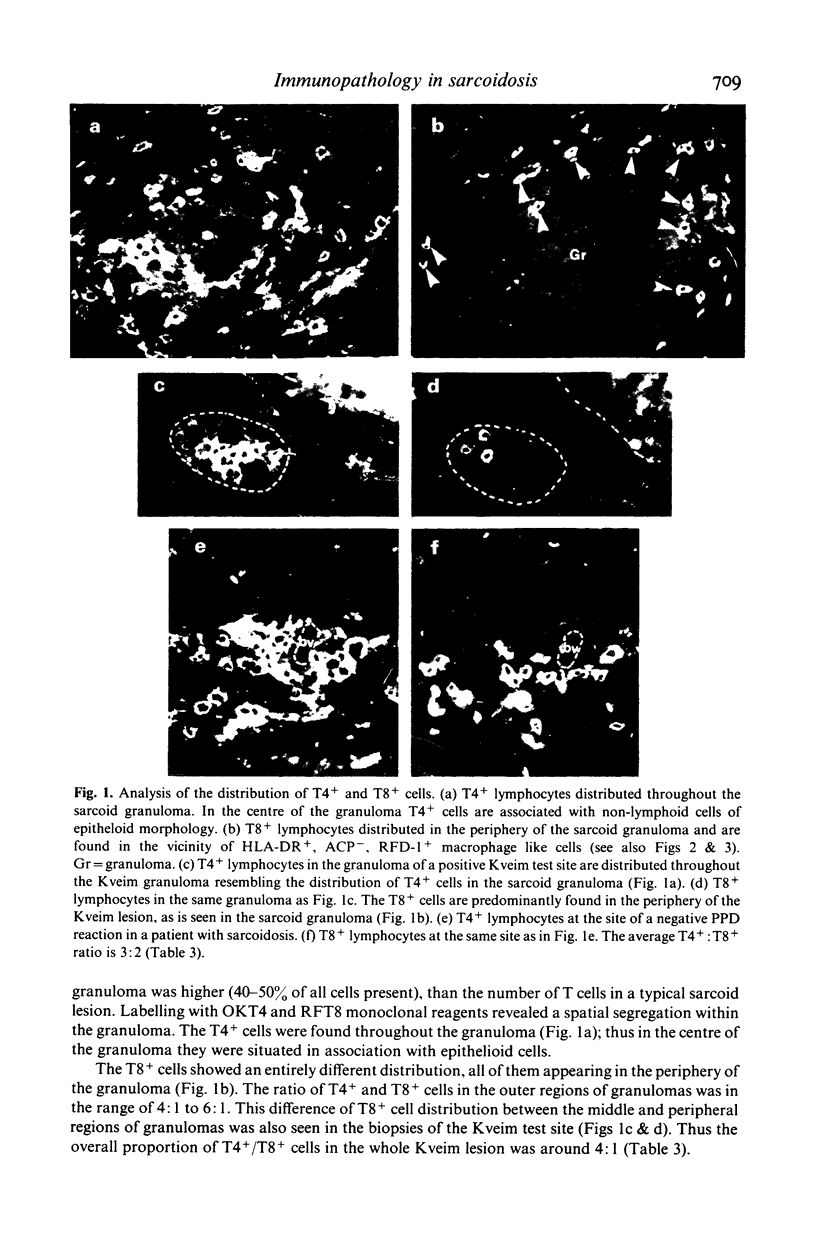




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O. The granulomatous inflammatory response. A review. Am J Pathol. 1976 Jul;84(1):164–192. [PMC free article] [PubMed] [Google Scholar]
- Balfour B. M., Drexhage H. A., Kamperdijk E. W., Hoefsmit E. C. Antigen-presenting cells, including Langerhans cells, veiled cells and interdigitating cells. Ciba Found Symp. 1981;84:281–301. doi: 10.1002/9780470720660.ch15. [DOI] [PubMed] [Google Scholar]
- Bjerke J. R., Krogh H. K., Matre R. In situ identification of mononuclear cells in cutaneous infiltrates in discoid lupus erythematosus, sarcoidosis and secondary syphilis. Acta Derm Venereol. 1981;61(5):371–380. [PubMed] [Google Scholar]
- Boros D. L. Granulomatous inflammations. Prog Allergy. 1978;24:183–267. doi: 10.1159/000401230. [DOI] [PubMed] [Google Scholar]
- Callard R. E., Smith C. M., Worman C., Linch D., Cawley J. C., Beverley P. C. Unusual phenotype and function of an expanded subpopulation of T cells in patients with haemopoietic disorders. Clin Exp Immunol. 1981 Mar;43(3):497–505. [PMC free article] [PubMed] [Google Scholar]
- JAMES D. G., THOMSON A. D., WILLCOX A. Erythema nodosum as a manifestation of sarcoidosis. Lancet. 1956 Aug 4;271(6936):218–221. doi: 10.1016/s0140-6736(56)90912-6. [DOI] [PubMed] [Google Scholar]
- James D. G. Immunology of sarcoidosis. Lancet. 1966 Sep 17;2(7464):633–635. doi: 10.1016/s0140-6736(66)91944-1. [DOI] [PubMed] [Google Scholar]
- James D. G., Neville E., Walker A. Immunology of sarcoidosis. Am J Med. 1975 Sep;59(3):388–394. doi: 10.1016/0002-9343(75)90397-6. [DOI] [PubMed] [Google Scholar]
- Janossy G., Bollum F. J., Bradstock K. F., McMichael A., Rapson N., Greaves M. F. Terminal transferase-positive human bone marrow cells exhibit the antigenic phenotype of common acute lymphoblastic leukemia. J Immunol. 1979 Oct;123(4):1525–1529. [PubMed] [Google Scholar]
- Janossy G., Thomas J. A., Goldstein G., Bollum F. J. The human thymic environment. Ciba Found Symp. 1981;84:193–214. [PubMed] [Google Scholar]
- Janossy G., Tidman N., Selby W. S., Thomas J. A., Granger S., Kung P. C., Goldstein G. Human T lymphocytes of inducer and suppressor type occupy different microenvironments. Nature. 1980 Nov 6;288(5786):81–84. doi: 10.1038/288081a0. [DOI] [PubMed] [Google Scholar]
- McMichael A. J., Pilch J. R., Galfré G., Mason D. Y., Fabre J. W., Milstein C. A human thymocyte antigen defined by a hybrid myeloma monoclonal antibody. Eur J Immunol. 1979 Mar;9(3):205–210. doi: 10.1002/eji.1830090307. [DOI] [PubMed] [Google Scholar]
- Modlin R. L., Hofman F. M., Meyer P. R., Sharma O. P., Taylor C. R., Rea T. H. In situ demonstration of T lymphocyte subsets in granulomatous inflammation: leprosy, rhinoscleroma and sarcoidosis. Clin Exp Immunol. 1983 Mar;51(3):430–438. [PMC free article] [PubMed] [Google Scholar]
- NIH conference. Pulmonary sarcoidosis: a disease characterized and perpetuated by activated lung T-lymphocytes. Ann Intern Med. 1981 Jan;94(1):73–94. doi: 10.7326/0003-4819-94-1-73. [DOI] [PubMed] [Google Scholar]
- Narayanan R. B., Bhutani L. K., Sharma A. K., Nath I. T cell subsets in leprosy lesions: in situ characterization using monoclonal antibodies. Clin Exp Immunol. 1983 Mar;51(3):421–429. [PMC free article] [PubMed] [Google Scholar]
- Papadimitriou J. M., Finlay-Jones J. M., Walters M. N. Surface characteristics of macrophages, epithelioid and giant cells using scanning electron microscopy. Exp Cell Res. 1973 Feb;76(2):353–362. doi: 10.1016/0014-4827(73)90387-x. [DOI] [PubMed] [Google Scholar]
- Poulter L. W. Antigen presenting cells in situ: their identification and involvement in immunopathology. Clin Exp Immunol. 1983 Sep;53(3):513–520. [PMC free article] [PubMed] [Google Scholar]
- Poulter L. W., Seymour G. J., Duke O., Janossy G., Panayi G. Immunohistological analysis of delayed-type hypersensitivity in man. Cell Immunol. 1982 Dec;74(2):358–369. doi: 10.1016/0008-8749(82)90036-3. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. Current concepts in immunology: Regulation of the immune response--inducer and suppressor T-lymphocyte subsets in human beings. N Engl J Med. 1980 Aug 14;303(7):370–373. doi: 10.1056/NEJM198008143030704. [DOI] [PubMed] [Google Scholar]
- Rowe D. J., Isenberg D. A., McDougall J., Beverley P. C. Characterization of polymyositis infiltrates using monoclonal antibodies to human leucocyte antigens. Clin Exp Immunol. 1981 Aug;45(2):290–298. [PMC free article] [PubMed] [Google Scholar]
- Ryan G. B., Spector W. G. Natural selection of long-lived macrophages in experimental granulomata. J Pathol. 1969 Oct;99(2):139–151. doi: 10.1002/path.1710990208. [DOI] [PubMed] [Google Scholar]
- Semenzato G., Pezzutto A., Chilosi M., Pizzolo G. Redistribution of T lymphocytes in the lymph nodes of patients with sarcoidosis. N Engl J Med. 1982 Jan 7;306(1):48–49. doi: 10.1056/NEJM198201073060114. [DOI] [PubMed] [Google Scholar]
- Sharma O. P., James D. G., Fox R. A. A correlation of in vivo delayed-type hypersensitivity with in vitro lymphocyte transformation in sarccidosis. Chest. 1971 Jul;60(1):35–37. doi: 10.1378/chest.60.1.35. [DOI] [PubMed] [Google Scholar]
- Stein H., Gerdes J., Mason D. Y. The normal and malignant germinal centre. Clin Haematol. 1982 Oct;11(3):531–559. [PubMed] [Google Scholar]
- THOMSON A. D. [The pathology of sarcoidosis]. Postgrad Med J. 1958 May;34(391):248–253. doi: 10.1136/pgmj.34.391.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A., Janossy G., Chilosi M., Pritchard J., Pincott J. R. Combined immunological and histochemical analysis of skin and lymph node lesions in histiocytosis X. J Clin Pathol. 1982 Mar;35(3):327–337. doi: 10.1136/jcp.35.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]