Abstract
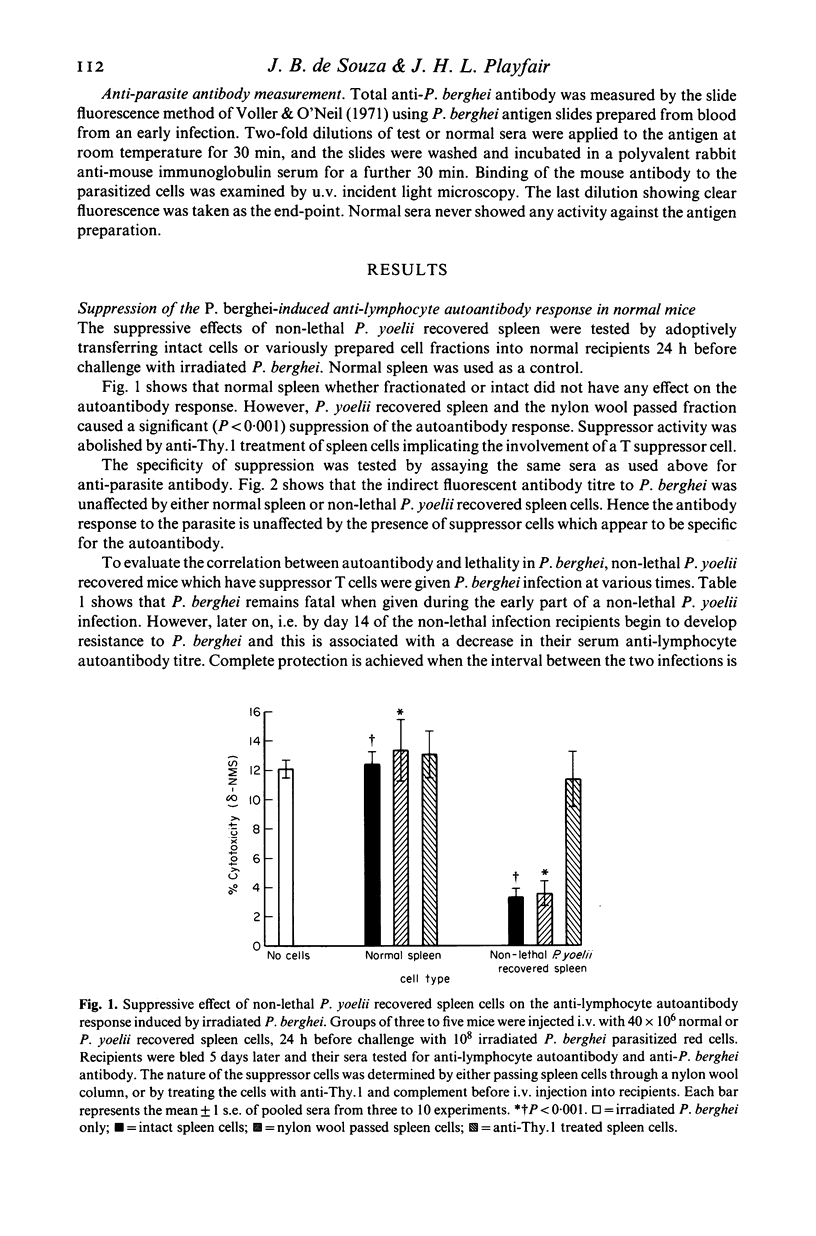
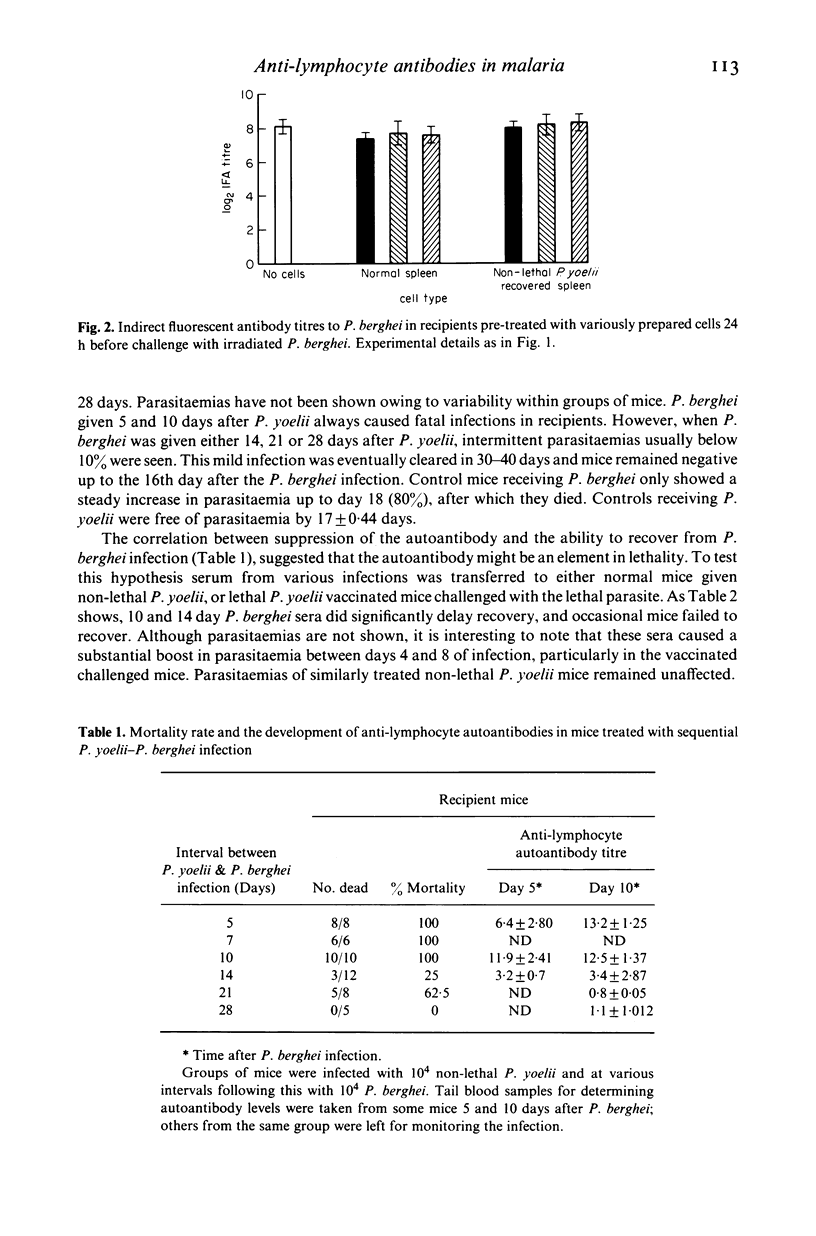
The anti-lymphocyte autoantibody response to irradiated lethal Plasmodium berghei malaria parasites in normal mice was significantly reduced when recipients were pre-treated with splenic T cells from mice recovered from a non-lethal Plasmodium yoelii infection. Suppression was specific for the autoantibody and did not affect the antibody response to the parasite. Experiments involving sequential P. yoelii-P. berghei infections in situ revealed that recovery from P. berghei was possible when the interval between the two infections was 14 days or more. This ability to recover from P. berghei correlated with a progressive reduction of anti-lymphocyte autoantibody suggesting a useful role for the suppressor cell. The possible link between suppressor cells and anti-lymphocyte autoantibodies in malaria is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdalla S. H., Kasili F. G., Weatherall D. J. The coombs direct antiglobulin test in Kenyans. Trans R Soc Trop Med Hyg. 1983;77(1):99–102. doi: 10.1016/0035-9203(83)90028-7. [DOI] [PubMed] [Google Scholar]
- Cantor H., Gershon R. K. Immunological circuits: cellular composition. Fed Proc. 1979 Jun;38(7):2058–2064. [PubMed] [Google Scholar]
- Cottrell B. J., Playfair J. H., De Souza B. J. Cell-mediated immunity in mice vaccinated against malaria. Clin Exp Immunol. 1978 Nov;34(2):147–158. [PMC free article] [PubMed] [Google Scholar]
- Gilbreath M. J., Pavanand K., MacDermott R. P., Wells R. A., Ussery M. A. Characterization of cold reactive lymphocytotoxic antibodies in malaria. Clin Exp Immunol. 1983 Feb;51(2):232–238. [PMC free article] [PubMed] [Google Scholar]
- Hutchings P., Cooke A. Analysis of the cellular interactions involved in the regulation of induced erythrocyte autoantibodies. Cell Immunol. 1981 Sep 15;63(2):221–227. doi: 10.1016/0008-8749(81)90001-0. [DOI] [PubMed] [Google Scholar]
- Izui S., Kobayakawa T., Louis J., Lambert P. H. Induction of thymocytotoxic autoantibodies after injection of bacterial lipopolysaccharides in mice. Eur J Immunol. 1979 Apr;9(4):338–341. doi: 10.1002/eji.1830090416. [DOI] [PubMed] [Google Scholar]
- Jayawardena A. N., Targett G. A., Leuchars E., Carter R. L., Doenhoff M. J., Davies A. J. T-cell activation in murine malaria. Nature. 1975 Nov 13;258(5531):149–151. doi: 10.1038/258149a0. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Krettli A. U., Nussenzweig R. Depletion of T and B lymphocytes during malarial infections. Cell Immunol. 1974 Sep;13(3):440–446. doi: 10.1016/0008-8749(74)90263-9. [DOI] [PubMed] [Google Scholar]
- Lelchuk R., Sprott V. M., Playfair J. H. Differential involvement of non-specific suppressor T cells in two lethal murine malaria infections. Clin Exp Immunol. 1981 Aug;45(2):433–438. [PMC free article] [PubMed] [Google Scholar]
- Playfair J. H., De Souza J. B., Cottrell B. J. Protection of mice against malaria by a killed vaccine: differences in effectiveness against P. yoelii and P. berghei. Immunology. 1977 Oct;33(4):507–515. [PMC free article] [PubMed] [Google Scholar]
- Playfair J. H., de Souza J. B. Lymphocyte traffic and lymphocyte destruction in murine malaria. Immunology. 1982 May;46(1):125–133. [PMC free article] [PubMed] [Google Scholar]
- Voller A., O'Neill P. Immunofluorescence method suitable for large-scale application to malaria. Bull World Health Organ. 1971;45(4):524–529. [PMC free article] [PubMed] [Google Scholar]
- Wedderburn N., Turk J. L., Hutt M. S. Chronic malarial infection in Balb/C mice. Effect on the immune response to sheep erythrocytes and histological changes in the liver and spleen. Trans R Soc Trop Med Hyg. 1975;69(5-6):468–472. doi: 10.1016/0035-9203(75)90099-1. [DOI] [PubMed] [Google Scholar]
- Wells R. A., Pavanand K., Zolyomi S., Permpanich B., MacDermott R. P. Loss of circulating T lymphocytes with normal levels of B and 'null' lymphocytes in Thai adults with malaria. Clin Exp Immunol. 1979 Feb;35(2):202–209. [PMC free article] [PubMed] [Google Scholar]
- Wells R. A., Pavanand K., Zolyomi S., Permpanich B., Macdermott R. P. Anti-lymphocytotoxic antibodies in sera of Thai adults infected with Plasmodium falciparum or Plasmodium vivax. Clin Exp Immunol. 1980 Mar;39(3):663–667. [PMC free article] [PubMed] [Google Scholar]
- de Souza J. B., Playfair J. H. Antilymphocyte autoantibody in lethal mouse malaria and its suppression by non-lethal malaria. Parasite Immunol. 1983 May;5(3):257–265. doi: 10.1111/j.1365-3024.1983.tb00742.x. [DOI] [PubMed] [Google Scholar]


