Abstract
A large number of cancer-associated gene products evoke immune recognition, but host reactions rarely impede disease progression. The weak immunogenicity of nascent tumors contributes to this failure in host defense. Therapeutic vaccines that enhance dendritic cell presentation of cancer antigens increase specific cellular and humoral responses, thereby effectuating tumor destruction in some cases. The attenuation of T cell activation by cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) further limits the potency of tumor immunity. In murine systems, the administration of antibodies that block CTLA-4 function inhibits the growth of moderately immunogenic tumors and, in combination with cancer vaccines, increases the rejection of poorly immunogenic tumors, albeit with a loss of tolerance to normal differentiation antigens. To gain a preliminary assessment of the biologic activity of antagonizing CTLA-4 function in humans, we infused a CTLA-4 blocking antibody (MDX-CTLA4) into nine previously immunized advanced cancer patients. MDX-CTLA4 stimulated extensive tumor necrosis with lymphocyte and granulocyte infiltrates in three of three metastatic melanoma patients and the reduction or stabilization of CA-125 levels in two of two metastatic ovarian carcinoma patients previously vaccinated with irradiated, autologous granulocyte–macrophage colony-stimulating factor-secreting tumor cells. MDX-CTLA4 did not elicit tumor necrosis in four of four metastatic melanoma patients previously immunized with defined melanosomal antigens. No serious toxicities directly attributable to the antibody were observed, although five of seven melanoma patients developed T cell reactivity to normal melanocytes. These findings suggest that CTLA-4 antibody blockade increases tumor immunity in some previously vaccinated cancer patients.
The formulation of genetic and biochemical strategies to identify cancer antigens yielded the unexpected discovery that tumor development frequently evokes immune recognition (1, 2). Cancer-associated gene products may stimulate T, B, and natural killer T (NKT) lymphocytes, natural killer cells, and phagocytes (3–7). Although the presence of brisk T cell infiltrates in human tumors is correlated with improved clinical outcomes, host responses in most cases are insufficient to inhibit disease progression (8–12).
One mechanism that may contribute to the failure of host defense is inadequate tumor antigen presentation (13). Cancer cells typically lack the expression of costimulatory molecules necessary to prime potent T lymphocyte responses directly, and dendritic cells infiltrating established tumors generally display limited maturation (14). Under these conditions, the induced tumor-reactive T cells manifest impaired functional capabilities. One strategy to ameliorate this defect in antigen presentation involves vaccination with irradiated tumor cells engineered to secrete granulocyte–macrophage colony-stimulating factor (GM-CSF) (15). Immunization elicits large numbers of activated CD11b+ dendritic cells that express high levels of B7-1, B7-2, MHC II, and CD1d (16). These recruited cells efficiently phagocytose and process dying tumor cells, migrate to regional lymph nodes, and stimulate tumor-specific lymphocytes (17, 18). CD4+ and CD8+ T cells, CD1d-restricted invariant NKT cells, and antibodies mediate protective immunity (15, 16, 19, 20). A phase I clinical trial using retroviral-mediated gene transfer to engineer autologous GM-CSF-secreting melanoma cells established the ability of this vaccination scheme to enhance cancer immunity in metastatic melanoma patients (21). A second therapeutic strategy to improve tumor antigen presentation involves the loading of cancer antigens, in a variety of formulations, onto ex vivo-expanded dendritic cells (22). Several early-stage clinical trials have also demonstrated the ability of this vaccination scheme to increase tumor immunity (23–27).
Although a minority of patients achieved durable clinical responses in these studies, most eventually succumbed to progressive disease. One mechanism that may limit the therapeutic potency of cancer vaccines is the attenuation of T cell function by cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) (28). Although the binding of B7-1 or B7-2 to CD28 provides an important costimulatory signal, the engagement of CTLA-4 by these ligands induces cell cycle arrest and diminished cytokine production (29–31). The development of a lethal lymphoproliferative disorder in young CTLA-4-deficient mice illuminates the pivotal role of CTLA-4 in immune homeostasis (32, 33). As CD4+, but not CD8+, T cell depletion reduces the autoimmune disease in these mice, the activities of CTLA-4 are essential for normal helper T cell regulation (34).
In contrast to the severe pathology characteristic of CTLA-4-deficient mice, transient CTLA-4 antibody blockade enhances antigen-specific T cell responses with limited toxicities. The injection of anti-CTLA-4 antibodies stimulates the rejection of moderately immunogenic murine tumors, and this activity may be potentiated with chemotherapy (35–38). Although CTLA-4 antibody blockade alone elicits minimal effects against poorly immunogenic tumors, concurrent vaccination with irradiated, GM-CSF-secreting tumor cells is highly efficacious in the B16 melanoma, SM1 breast carcinoma, and transgenic adenocarcinoma of the mouse prostate (TRAMP) carcinoma models (39–41). Cytotoxic T cells are critical for tumor destruction, but the augmented anticancer response may be associated with the loss of tolerance to normal differentiation antigens, culminating in autoimmune vitiligo or prostatitis (42).
To gain a preliminary assessment of the biologic activity of antagonizing CTLA-4 function in humans, we administered the CTLA-4 blocking antibody MDX-CTLA-4 to nine previously vaccinated metastatic melanoma or ovarian carcinoma patients.
Materials and Methods
Clinical Protocols.
The phase I studies of vaccination with irradiated, autologous melanoma or ovarian carcinoma cells engineered to secrete GM-CSF by adenoviral-mediated gene transfer will be reported elsewhere. The phase I study of vaccination with autologous dendritic cells engineered to express gp100 and MART-1 by adenoviral-mediated gene transfer will also be presented separately. The studies of vaccination with GM2 ganglioside admixed with QS-21 and immunization with a modified gp100 peptide plus IL-2 have been described (43, 44).
Patients 1–6 were enrolled in the Medarex-sponsored phase I trial MDX-CTLA-4-02 that was approved by the Dana–Farber Partners Cancer Care Institutional Review Board. Patients were eligible for this study if they had surgically unresectable stage III or stage IV malignant melanoma, disease progression, a life expectancy of at least 12 weeks, adequate end organ function, stable analgesic therapy, and a Karnofsky performance status of at least 60%. Patients were excluded if they used corticosteroids or had a second malignancy other than treated nonmelanoma skin cancer or superficial bladder cancer, autoimmune disease, active infection, or hypersensitivity to kanamycin. Patients 7–9 were treated on a Dana–Farber Partners Cancer Care-initiated trial of MDX-CTLA-4 infusion for metastatic melanoma, metastatic ovarian carcinoma, metastatic nonsmall cell lung carcinoma, or acute myelogenous leukemia patients previously vaccinated with irradiated, autologous, GM-CSF-secreting tumor cells. (The entire study, which will involve 16 patients, will be reported separately after its completion.) All patients provided written informed consent before enrollment in each clinical trial.
MDX-CTLA-4 is a human IgG1 antibody obtained from transgenic HuMAb mice, strain HC2/Kco7 (Medarex), immunized with the extracellular domain of CTLA-4. The antibody blocks binding of B7-1 Ig and B7-2 Ig to CTLA-4. MDX-CTLA-4 was drawn through a 0.22-μm filter and diluted in normal saline to a concentration of 2.5 mg/ml for administration. A test dose of 0.2 mg in 10 ml of normal saline was infused i.v. over 10 min to identify potential hypersensitivity reactions. The remainder of the 3 mg/kg MDX-CTLA-4 single dose was then delivered over 90 min with a volumetric pump. Patients were seen three times daily, four times weekly, and at monthly intervals thereafter for routine clinical, laboratory, and radiographic evaluation.
Pathology.
Tissues were fixed in 10% neutral buffered formalin, processed routinely, and embedded in paraffin. Immunohistochemistry was performed by using standard techniques with monoclonal antibodies to CD4, CD8, CD20, and Ig-κ.
Results
Patient Characteristics.
Seven metastatic melanoma and two ovarian carcinoma patients received MDX-CTLA-4 therapy between November 2000 and October 2002 (Table 1). The melanoma patients were all males with a median age of 49 years (range of 31–58 years). The average interval between the initial diagnosis of melanoma and study entry was 5 years (range of 3–9 years). Four patients received adjuvant therapies for early-stage disease (α-IFN, n = 3; vaccination with GM2 ganglioside admixed with QS-21, n = 1; radiation, n = 1). Nonimmunologic treatments for metastatic disease before enrollment were surgery (n = 4), radiation therapy (n = 2), chemotherapy (n = 3), and proteasome inhibitor (n = 1). The two ovarian carcinoma patients received multiple chemotherapies for relapsing disease throughout the 3–4 years before study enrollment.
Table 1.
Patient characteristics
| Patient | Age | Diagnosis | Treatment 1 | Recurrence | Treatment 2 | Treatment 3 | Treatment 4 | MDX-CTLA-4 | Metastases |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 43 | Skin (MM) 1995 | Surgery | Parotid, lung 1998 | GVAX 7/98–2/99 | Chemo 2/99–9/99 | PS341 4/00–6/00 | 1/01 | CNS, lung, abdomen, skin |
| 2 | 56 | LN (MM) 1992 | Surgery, α-IFN | SC, perirenal, LN, 1998 | GVAX 6/98–11/99 | — | — | 1/01 | LN, bone, perirenal |
| 3 | 43 | LN (MM) 1997 | Surgery, α-IFN | LN, intestine 1998 | Surgery 12/98 | DC 4/00–9/00 | — | 11/00 | LN |
| 4 | 58 | Skin (MM) 1998 | Surgery, XRT | Skin, lung 2000 | DC 5/00–8/00 | XRT 8/00 | — | 1/01 | CNS, lung |
| 5 | 49 | Skin, LN (MM) 1998 | Surgery, α-IFN | SC 2000 | DC 11/00–2/00 | — | — | 4/01 | SC, lung, liver, LN |
| 6 | 52 | SC (MM) 1997 | Surgery, GM2 | LN, lung 2000 | gp100 12/00–3/01 | Chemo 4/01–6/01 | — | 7/01 | Lung, LN |
| 7 | 31 | Skin (MM) 1994 | Surgery | LN, lung, SC, CNS, 1996 | Surgery, chemo, 8/96 | GVAX 7/98–10/98 | Chemo, XRT 1/99–6/99, 2/01–10/01 | 9/02 | SC |
| 8 | 38 | Pelvis (OV) 1998 | Surgery, chemo | Lung, pelvis 7/99 | Chemo 8/99–12/99 | MUC-1 4/00–6/00 | Chemo, GVAX, CI-1033, 6/00, 4/01, 2/02 | 6/02 10/02 | Lung, pelvis, retroperitone |
| 9 | 65 | PER (OV) 1999 | Chemo | PER 2001 | Chemo 9/01–12/01 | Chemo 2/02–5/02 | GVAX 6/02–8/02 | 9/02 | PER |
MM, malignant melanoma; OV, ovarian carcinoma; SC, subcutaneous tissue; LN, lymph node; PER, peritoneum; GM2, ganglioside + QS-21; GVAX, irradiated, autologous tumor cells engineered to secrete GM-CSF; DC, dendritic cells engineered to express gp100 and MART-1; gp100, peptide plus IL-2; MUC-1, MUC-1 conjugated to KLH plus QS-21; XRT, radiation therapy; PS341, proteosome inhibitor; CI-1033, epidermal growth factor kinase inhibitor.
All nine subjects participated in phase I vaccine studies for metastatic disease before entry into the MDX-CTLA-4 trial. Three melanoma and both ovarian carcinoma patients were immunized with irradiated, autologous tumor cells engineered to secrete GM-CSF by adenoviral-mediated gene transfer (patient 8 also received a MUC-1 vaccine). Three melanoma patients were immunized with autologous dendritic cells engineered to express gp100 and MART-1 by adenoviral-mediated gene transfer. One melanoma patient was vaccinated with a modified gp100 peptide and high-dose IL-2.
MDX-CTLA-4 Toxicities.
A single dose of the human MDX-CTLA-4 antibody (3 mg/kg) was administered i.v. over 1.5 h. There was one acute hypersensitivity reaction characterized by mild hypotension and nausea during the infusion; this was easily controlled with antihistamines, and the treatment was completed uneventfully. Five patients developed transient grade 1–2 constitutional symptoms consisting of myalgias, arthralgias, anorexia, fatigue, nasal congestion, and nonproductive cough 2–7 days after the infusion; in one case, the syndrome recurred intermittently for several months. One patient with hepatic metastases manifested a transient grade 3 liver function test abnormality. Otherwise, there were no significant renal, pulmonary, cardiac, hematologic, gastrointestinal, or neurologic toxicities directly attributable to the antibody.
Autoimmune Reactions.
MDX-CTLA-4 stimulated low titers of autoantibodies that persisted for 1–2 months without clinical evidence of autoimmune disease in four patients (Table 2). These included antinuclear antibodies (speckled pattern at 1:80 or 1:160 dilutions), antithyroglobulin antibodies (212, normal <60), and rheumatoid factors (28, normal <15). Four subjects mounted short-lived (24 h to 2 weeks) increases in circulating neutrophil counts (2- to 4-fold elevations).
Table 2.
Biologic activity of CTLA-4 antibody blockade
| Patient | Prior vaccine | Autoantibodies | Δ Neut | Melanocyte reactivity | Antitumor effects |
|---|---|---|---|---|---|
| 1 | GVAX | None | 2 | Skin | Extensive hemorrhagic necrosis with granulocytes and lymphocytes |
| 2 | GVAX | ANA 1:160 | 4 | Skin retina | Extensive necrosis with granulocytes and lymphocytes; vasculopathy |
| 3 | DC | ANA 1:80, α-TG 212 | 3 | Skin | CD8+ T cell infiltrate; no tumor necrosis |
| 4 | DC | RF 28 | 0 | Skin | CD8+ T cell infiltrate; no tumor necrosis |
| 5 | DC | None | 2.5 | Skin | No tumor necrosis; absent infiltrate |
| 6 | GM2, gp100 | None | 0 | No | Not done |
| 7 | GVAX | None | 0 | No | Extensive necrosis with granulocytes and lymphocytes; vasculopathy |
| 8 | MUC-1, GVAX | None | 0 | No | CA-125 reduction |
| 9 | GVAX | ANA 1:160 | 0 | No | CA-125 stabilization |
GVAX, irradiated, autologous tumor cells engineered to secrete GM-CSF; DC, dendritic cells engineered to express gp100 and MART-1; gp100, peptide plus IL-2; MUC-1, MUC-1 conjugated to KLH plus QS-21; ANA, antinuclear antibodies; α-TG, antithyroglobulin antibodies; RF, rheumatoid factor; Δ Neut, fold increase in neutrophils.
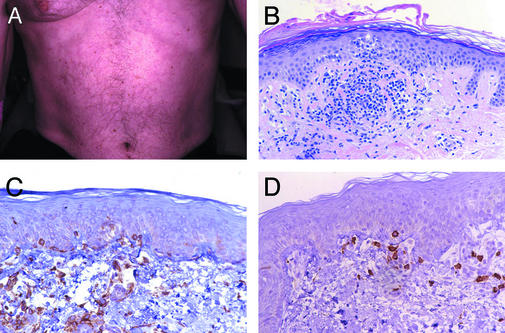
All of the melanoma patients developed an asymptomatic, grade 1 reticular and erythematous rash on the trunk and extremities between 3 days and 3 weeks after MDX-CTLA-4 infusion (Fig. 1A). Punch biopsies of the skin in five of the seven subjects revealed prominent peri-vascular T cell infiltrates in the superficial dermis that extended into the epidermis (Fig. 1B). CD4+ and CD8+ T cells were found apposed to dying melanocytes in these sections (Fig. 1 C and D), although vitiligo was not clinically evident. Mild, focal hypopigmentation of the retinal pigmented epithelium was also detected by ophthalmologic examination in one patient, but this was not associated with a change in visual acuity. One ovarian carcinoma patient (no. 8) developed a transient erythematous rash on the face and trunk 2 weeks after infusion. Skin biopsy demonstrated perivascular T cell infiltrates in the superficial dermis, but no host reactivity toward melanocytes (not shown). Changes similar to these may be observed in hypersensitivity responses or some connective tissue diseases.
Figure 1.
MDX-CTLA-4 stimulated melanocyte immune recognition. (A) Reticular erythematous rash. (B) Perivascular lymphocyte infiltrate extending into epidermis with interface dermatitis. (C) CD4+ T cells apposed to dying melanocytes. (D) CD8+ T cells apposed to dying melanocytes. (Magnification: ×125, B; ×250, C and D.)
Antitumor Effects.
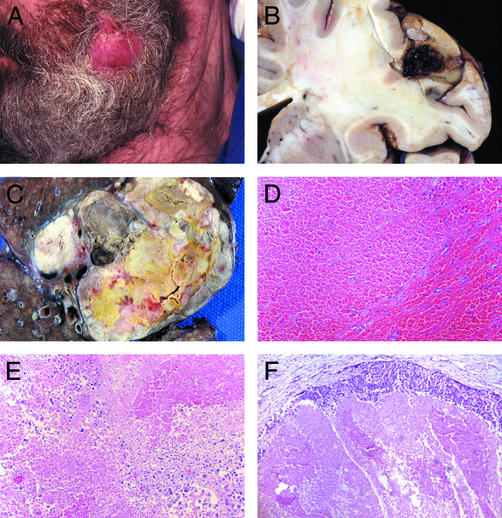
MDX-CTLA-4 elicited extensive tumor necrosis with immune infiltrates in the three melanoma patients previously vaccinated with irradiated, autologous GM-CSF-secreting tumor cells. Patient 1 harbored metastases in the CNS, lungs, abdomen, and soft tissues at study entry. One month after MDX-CTLA-4 administration, a distinct change in clinical status was noted. A s.c. nodule became acutely inflamed (Fig. 2A), and facial twitching, slurred speech, impaired coordination, and weakness developed shortly thereafter. Magnetic resonance imaging revealed an increase in the gadolinium uptake of multiple brain lesions, suggesting an alteration in blood flow. Several cord compressions were detected in the cervical and thoracic spine, and large visceral metastases were present in the abdomen and lung. The patient deteriorated rapidly and died 6 days later, likely from CNS disease. Unexpectedly, marked hemorrhagic tumor necrosis was found at gross autopsy in numerous brain, epidural, and visceral metastases (Fig. 2 B and C). Histopathologic examination disclosed extensive tumor destruction (at least 90%) with hemorrhage (Fig. 2 D and E). While a rim of viable tumor cells persisted in each lesion, this was accompanied by a granulocyte and lymphocyte reaction (Fig. 2F).
Figure 2.
MDX-CTLA-4 induced hemorrhagic tumor necrosis in vaccinated patient 1. (A) Inflamed s.c. nodule. (B) Necrotic brain metastasis. (C) Necrotic lung metastasis. (D and E) Hemorrhagic tumor necrosis. (F) Rim of viable tumor with granulocytes and lymphocytes. (Magnification: ×250, D–F.)
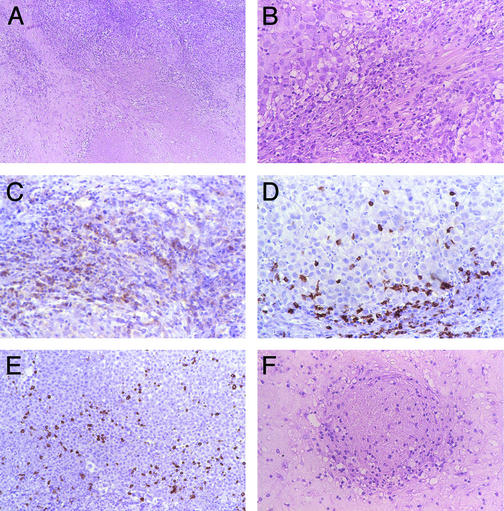
Patient 2 manifested recurrent episodes of grade 2 constitutional symptoms beginning 1 month after MDX-CTLA-4 infusion. A biopsy of a mediastinal mass revealed extensive tumor necrosis with lymphocyte and granulocyte infiltrates (Fig. 3 A and B). Neutrophils were more prominent than eosinophils in the reaction. Immunohistochemistry disclosed the presence of CD4+ and CD8+ T cells and CD20+ B lymphocytes producing Ig (Fig. 3 C–E). The lesion was completely resected 2 months later, and pathologic analysis demonstrated dense fibrosis, extensive necrosis, and an ongoing lymphocyte and granulocyte response (not shown). A vasculopathy characterized by a circumferential lymphoid infiltrate in the wall of an occluded blood vessel was also noted (Fig. 3F); tumor necrosis appeared spatially related to the vessel damage. Three months afterward, a zygomatic arch metastasis recurred. Chemotherapy with cisplatin and dacarbazine induced substantial tumor regression that persisted for 18 months.
Figure 3.
MDX-CTLA-4 induced extensive tumor necrosis in vaccinated patient 2. (A and B) Tumor necrosis with granulocytes and lymphocytes. (C) CD4+ T cells. (D) CD8+ T cells. (E) CD20+ B cells. (F) Vasculopathy with perivascular and intramural lymphoid infiltrates associated with luminal thrombosis. (Magnification: ×125, A; ×250, B–F.)
Patient 7 developed inflammation in a large s.c. mass 3 weeks after MDX-CTLA-4 infusion. The lesion was excised at 2 months, and pathologic examination similarly revealed extensive tumor necrosis and fibrosis with lymphocyte and granulocyte infiltrates. Moreover, perivascular lymphoid aggregates and infiltration of the vessel wall associated with thrombosis was again observed (data not shown).
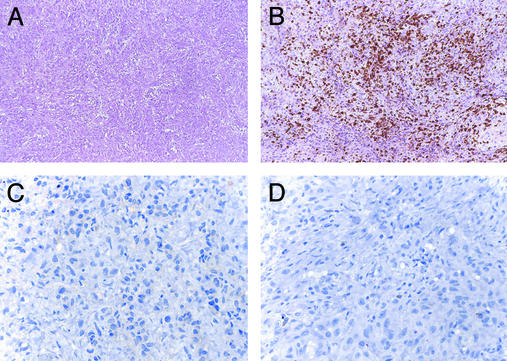
CTLA-4 antibody blockade evoked less significant antitumor effects in the four melanoma patients previously immunized with defined melanosomal antigens. Patient 3 underwent a resection of an enlarging mediastinal mass 7 months after MDX-CTLA-4 infusion. Pathologic study revealed a dense lymphocyte infiltrate without tumor necrosis (Fig. 4A). Immunohistochemistry disclosed the presence of CD8+ T, but not CD4+ T or CD20+ B, cells (Fig. 4 B–D). Although a brief period of stable disease followed treatment with cisplatin and dacarbazine, brain metastases developed shortly thereafter. Patient 4 showed a comparable CD8+ T cell infiltrate without tumor necrosis in a lymph node metastasis resected 2 months after antibody administration. Subsequent chemotherapy similarly did not achieve a response, and the patient died 10 months after study entry. Patient 5 failed to develop lymphoid infiltrates or tumor necrosis in a s.c. metastasis resected 2 months after MDX-CTLA-4 administration (data not shown); follow-up chemotherapy was ineffective. Lastly, although patient 6 did not undergo a biopsy after MDX-CTLA-4 infusion, his clinical course was characterized by steady tumor progression that proved unresponsive to chemotherapy.
Figure 4.
MDX-CTLA-4 induced CD8+ T cell infiltrates but no tumor necrosis in vaccinated patients 3 and 4. (A) Lymphocyte infiltrate without tumor destruction. (B) CD8+ T cells. (C) CD4+ T cells. (D) CD20+ B cells. (Magnification: ×250, A and B; ×500, C and D.)
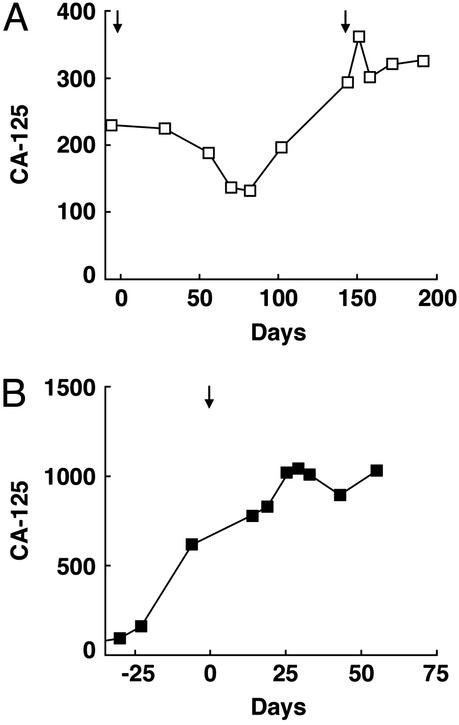
Although tumor biopsies could not be obtained in the two ovarian carcinoma patients after MDX-CTLA-4 infusion, the antibody elicited clear changes in blood CA-125 levels. This glycoprotein antigen is shed from the surface of ovarian carcinoma cells, thereby serving as a useful marker of disease status (45). Patient 8 showed a 43% reduction in CA-125 values (from 230 to 132) beginning 2 months after antibody infusion; although this response was not maintained, a second infusion of MDX-CTLA-4 stabilized CA-125 levels for 2 months (Fig. 5A). Patient 9 achieved a plateau in CA-125 values 1 month after antibody infusion (concomitant with a reduction in ascites and pain), despite rapidly rising levels before treatment (Fig. 5B).
Figure 5.
MDX-CTLA-4 induced alterations in the circulating ovarian carcinoma tumor marker CA-125. (A) Patient 8. (B) Patient 9. Arrows indicate MDX-CTLA-4 infusions.
Discussion
This phase I clinical investigation was undertaken in an effort to obtain a preliminary assessment of the biologic activity and toxicity of MDX-CTLA-4 in previously vaccinated metastatic melanoma and ovarian carcinoma patients. The study was motivated by compelling preclinical data indicating that the combination of CTLA-4 antibody blockade and cancer vaccination stimulated greater levels of antitumor immunity than either approach alone. Because the combination treatment also provoked a loss of tolerance to normal differentiation antigens, the risk of serious toxicities to patients was of some concern. Hence, we initially elected to administer CTLA-4 antibody blockade to previously vaccinated cancer patients.
Our initial results suggest that a single infusion of MDX-CTLA-4 may be safely delivered in this clinical setting. The generation of low titers of autoantibodies shows that the therapy may at least partially compromise systemic tolerance, but no evidence for autoimmune disease was noted. The melanoma patients developed a reticular and erythematous rash caused by perivascular lymphoid aggregates in the superficial dermis that extended into the epidermis. Both CD4+ and CD8+ T cells were juxtaposed with dying melanocytes in the skin, and one patient manifested focal hypopigmentation of the retinal pigmented epithelium as well. Although these findings demonstrate a loss of tolerance to melanocyte differentiation antigens, neither vitiligo nor alterations in visual acuity ensued, likely reflecting incomplete melanocyte destruction. Although the preclinical studies delineated a critical role for CD8+ T cells in mediating depigmentation (42), the data presented here suggest that CD4+ T cells may also contribute to melanocyte recognition.
MDX-CTLA-4 elicited antitumor effects in five of five patients previously immunized with irradiated, autologous GM-CSF-secreting tumor cells, whereas minimal tumor destruction was noted in the four patients previously immunized with defined melanosomal antigens. These preliminary findings raise the possibility that specific characteristics of preexisting tumor immunity may influence the response to subsequent CTLA-4 antibody blockade. The results may also be consistent with experiments showing that CTLA-4 antibody blockade elicits potent antitumor effects against moderately immunogenic, but not poorly immunogenic, murine tumors (35–41).
The mechanisms underlying the ability of CTLA-4 antibody blockade to increase tumor immunity remain to be clarified. Recent investigations reveal that CTLA-4 traffics to the immunologic synapse in response to T cell activation, thereby delivering an attenuating signal (46, 47). In immunized patients, tumor-reactive memory or effector T cells encountering antigen-loaded dendritic cells in the periphery or secondary lymphoid tissues may be the targets for CTLA-4 antibody blockade. Alternatively, MDX-CTLA-4 may modulate the activities of regulatory T cells that constitutively express surface CTLA-4 (48). In either or both cases, the relative importance of CTLA-4 blockade in augmenting effector T cell function versus modifying the affinity and/or breadth of T cell tumor recognition remains to be delineated.
Pathologic analysis of the metastases resected after MDX-CTLA-4 infusion disclosed several pathways that contributed to tumor destruction. The autopsy of patient 1 revealed striking hemorrhagic necrosis in all of the lesions examined. Tumor blood vessels were severely damaged in these masses, resulting in extensive ischemic necrosis and some bleeding. A rim of viable tumor was still present, but it was infiltrated with granulocytes and lymphocytes. Similar pathologic features were originally described in response to Coley's toxins (49), and tumor necrosis factor was later identified as one mediator of the reaction (50). Although the mechanisms underlying hemorrhagic necrosis are not yet fully defined, both soluble factors and leukocytes participate in vessel destruction (51). Indeed, a striking circumferential lymphoid infiltrate was detected in occluded tumor blood vessels in patients 2 and 7. As this mechanism of tumor destruction does not involve a rapid reduction in tumor volume, MDX-CTLA-4 may not prove clinically useful, however, in the setting of large CNS metastases.
An unexpected finding was the prominent effect of CTLA-4 antibody blockade on neutrophil responses. MDX-CTLA-4 induced significant increases in circulating neutrophils, and robust neutrophil infiltrates were associated with tumor necrosis. A primary role for neutrophils in mediating tumor destruction was previously suggested by experiments characterizing the host reaction to tumor cells engineered to secrete G-CSF (52). As T cells from CTLA-4-deficient mice show enhanced secretion of multiple cytokines including GM-CSF (33, 53), the recruitment of neutrophils may be secondary to T cell activation.
The serial biopsies of the mediastinal mass from patient 2 suggested the possibility of the evolution of a coordinated cellular and humoral antitumor response. Immunohistochemistry disclosed CD4+ and CD8+ T cells and CD20+ B cells producing Ig. Although the preclinical studies underscored the importance of cytotoxic T cells in effectuating tumor destruction (42), these results suggest that a broader lymphocyte reaction may be involved. Indeed, CD8+ T cells (without CD4+ and CD20+ lymphocytes) were detected in the metastases of two patients previously immunized with melanosomal antigens; however, neither lesion manifested tumor necrosis. More detailed investigations of the functions of helper T and B cells in the antitumor effects of CTLA-4 antibody blockade are warranted.
Overall, the findings reported here should stimulate more extensive clinical evaluation of the combination of tumor vaccines and MDX-CTLA-4. Although the preclinical experiments tested concurrent administration, this study illustrates that the temporal separation of immunization and antibody blockade may also elicit important antitumor effects. Because our previous reports of GM-CSF-secreting melanoma vaccines similarly revealed the induction of a vasculopathy and of granulocyte, CD4+, CD8+, and CD20+ lymphocyte infiltrates effectuating extensive tumor necrosis and fibrosis (21, 54), the current results suggest that MDX-CTLA-4 may amplify a long-lived memory response in patients (55, 56). Future comparison of the relative toxicity and immunogenicity of concurrent versus sequential combination therapy should provide a deeper understanding of the mechanisms limiting effective tumor immunity.
Acknowledgments
We thank the Connell–O'Reilly Laboratory for excellent processing of patient material and Christine Sheehan and Esther Brisson (Albany Medical College, Albany, NY) for excellent help with the histologic specimens. This study was supported by the Berlex Oncology Foundation, National Institutes of Health Grants CA78880 (to F.S.H.), CA74886, and CA39542 (to G.D.), the Leukemia and Lymphoma Society Score Award in Acute Myeloid Leukemia (to G.D.), the Cancer Research Institute/Partridge Foundation Clinical Investigator Award, the Cancer Research Institute Melanoma Initiative (to G.D.), and Medarex. G.D. and R.J.S. are Clinical Scholars of the Leukemia and Lymphoma Society.
Abbreviations
- CTLA-4
cytotoxic T lymphocyte-associated antigen 4
- GM-CSF
granulocyte–macrophage colony-stimulating factor.
References
- 1.Boon T, van der Bruggen P. J Exp Med. 1996;183:725–729. doi: 10.1084/jem.183.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Old L, Chen Y-T. J Exp Med. 1998;187:1163–1167. doi: 10.1084/jem.187.8.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sahin U, Tureci O, Schmitt H, Cochlovius B, Johannes T, Schmits R, Stenner F, Luo G R, Schobert I, Pfreundschuh M. Proc Natl Acad Sci USA. 1995;92:11810–11813. doi: 10.1073/pnas.92.25.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng G, Wang X, Robbins P, Rosenberg S, Wang R-F. Proc Natl Acad Sci USA. 2001;98:3964–3969. doi: 10.1073/pnas.061507398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jäger E, Nagata Y, Gnjatic S, Wada H, Stockert E, Karbach J, Dunbar P, Lee S, Jungbluth A, Jäger D, et al. Proc Natl Acad Sci USA. 2000;97:4760–4765. doi: 10.1073/pnas.97.9.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauer S, Groh V, Wu J, Steinle A, Phillips J H, Lanier L L, Spies T. Science. 1999;285:727–729. [PubMed] [Google Scholar]
- 7.Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein K H, Spies T. Proc Natl Acad Sci USA. 1999;96:6879–6884. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark W, Elder D, Guerry D, Braitman L, Trock B, Schultz D, Synnestvedt M, Halpern A. J Natl Cancer Inst. 1989;81:1893–1904. doi: 10.1093/jnci/81.24.1893. [DOI] [PubMed] [Google Scholar]
- 9.Clemente C, Mihm M, Bufalino R, Zurrida S, Collini P, Cascinelli N. Cancer (Philadelphia) 1996;77:1303–1310. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 10.Mihm M, Clemente C, Cascinelli N. Lab Invest. 1996;74:43–47. [PubMed] [Google Scholar]
- 11.Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. Cancer Res. 1998;58:3491–3494. [PubMed] [Google Scholar]
- 12.Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, Suzuki Y, Shintaku I, Nagura H, Ohtani H. Cancer Res. 2001;61:5132–5136. [PubMed] [Google Scholar]
- 13.Banchereau J, Steinman R. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 14.Bell D, Chomarat P, Broyles D, Netto G, Harb G, Lebecque S, Valladeau J, Davoust J, Palucka K, Banchereau J. J Exp Med. 1999;190:1417–1425. doi: 10.1084/jem.190.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan R C. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mach N, Gillessen S, Wilson S B, Sheehan C, Mihm M, Dranoff G. Cancer Res. 2000;60:3239–3246. [PubMed] [Google Scholar]
- 17.Huang A Y, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 18.Shen Z, Reznikoff G, Dranoff G, Rock K. J Immunol. 1997;158:2723–2730. [PubMed] [Google Scholar]
- 19.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll H, Levitsky H. J Exp Med. 1998;188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reilly R, Machiels J-P, Emens L, Ercolini A, Okoye F, Lei R, Weintraub D, Jaffee E. Cancer Res. 2001;61:880–883. [PubMed] [Google Scholar]
- 21.Soiffer R, Lynch T, Mihm M, Jung K, Rhuda C, Schmollinger J, Hodi F, Liebster L, Lam P, Mentzer S, et al. Proc Natl Acad Sci USA. 1998;95:13141–13146. doi: 10.1073/pnas.95.22.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young J W, Inaba K. J Exp Med. 1996;183:7–11. doi: 10.1084/jem.183.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu F, Benike C, Fagnoni F, Liles T, Czerwinski D, Taidi B, Engleman E, Levy R. Nat Med. 1996;2:52–58. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 24.Nestle F, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 25.Banchereau J, Palucka A K, Dhodapkar M, Burkeholder S, Taquet N, Rolland A, Taquet S, Coquery S, Wittkowski K M, Bhardwaj N, et al. Cancer Res. 2001;61:6451–6458. [PubMed] [Google Scholar]
- 26.Heiser A, Coleman D, Dannull J, Yancey D, Maurice M A, Lallas C D, Dahm P, Niedzwiecki D, Gilboa E, Vieweg J. J Clin Invest. 2002;109:409–417. doi: 10.1172/JCI14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuler-Thurner B, Schultz E S, Berger T G, Weinlich G, Ebner S, Woerl P, Bender A, Feuerstein B, Fritsch P O, Romani N, Schuler G. J Exp Med. 2002;195:1279–1288. doi: 10.1084/jem.20012100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chambers C A, Kuhns M S, Egen J G, Allison J P. Annu Rev Immunol. 2001;19:565–594. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 29.Thompson C B, Allison J P. Immunity. 1997;7:445–450. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- 30.Doyle A M, Mullen A C, Villarino A V, Hutchins A S, High F A, Lee H W, Thompson C B, Reiner S L. J Exp Med. 2001;194:893–902. doi: 10.1084/jem.194.7.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salomon B, Bluestone J A. Annu Rev Immunol. 2001;19:225–252. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 32.Waterhouse P, Penninger J M, Timms E, Wakeham A, Shahinian A, Lee K P, Thompson C B, Griesser H, Mak T W. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 33.Tivol E A, Borriello F, Schweitzer A N, Lynch W P, Bluestone J A, Sharpe A H. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 34.Chambers C A, Sullivan T J, Allison J P. Immunity. 1997;7:885–895. doi: 10.1016/s1074-7613(00)80406-9. [DOI] [PubMed] [Google Scholar]
- 35.Leach D R, Krummel M F, Allison J P. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y F, Zou J P, Mu J, Wijesuriya R, Ono S, Walunas T, Bluestone J, Fujiwara H, Hamaoka T. Cancer Res. 1997;57:4036–4041. [PubMed] [Google Scholar]
- 37.Kwon E D, Hurwitz A A, Foster B A, Madias C, Feldhaus A L, Greenberg N M, Burg M B, Allison J P. Proc Natl Acad Sci USA. 1997;94:8099–8103. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mokyr M B, Kalinichenko T, Gorelik L, Bluestone J A. Cancer Res. 1998;58:5301–5304. [PubMed] [Google Scholar]
- 39.van Elsas A, Hurwitz A, Allison J. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hurwitz A, Yu T, Leach D, Allison J. Proc Natl Acad Sci USA. 1998;95:10067–10071. doi: 10.1073/pnas.95.17.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hurwitz A A, Foster B A, Kwon E D, Truong T, Choi E M, Greenberg N M, Burg M B, Allison J P. Cancer Res. 2000;60:2444–2448. [PubMed] [Google Scholar]
- 42.van Elsas A, Sutmuller R P, Hurwitz A A, Ziskin J, Villasenor J, Medema J P, Overwijk W W, Restifo N P, Melief C J, Offringa R, Allison J P. J Exp Med. 2001;194:481–489. doi: 10.1084/jem.194.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Livingston P, Wong G, Adluri S, Tao Y, Padavan M, Parente R, Hanlon C, Calves M, Helling F, Ritter G, et al. J Clin Oncol. 1994;12:1036–1044. doi: 10.1200/JCO.1994.12.5.1036. [DOI] [PubMed] [Google Scholar]
- 44.Rosenberg S, Yang J, Schwartzentruber D, Hwu P, Marincola F, Topalian S, Restifo N, Dudley M, Schwarz S, Spiess P, et al. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jacobs I. Gynecol Oncol. 1994;55:S22–S27. doi: 10.1006/gyno.1994.1336. [DOI] [PubMed] [Google Scholar]
- 46.Egen J G, Allison J P. Immunity. 2002;16:23–235. doi: 10.1016/s1074-7613(01)00259-x. [DOI] [PubMed] [Google Scholar]
- 47.Darlington P J, Baroja M L, Chau T A, Siu E, Ling V, Carreno B M, Madrenas J. J Exp Med. 2002;195:1337–1347. doi: 10.1084/jem.20011868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shevach E M, McHugh R S, Piccirillo C A, Thornton A M. Immunol Rev. 2001;182:58–67. doi: 10.1034/j.1600-065x.2001.1820104.x. [DOI] [PubMed] [Google Scholar]
- 49. Nauts, H., Fowler, G. & Bogatko, F. (1953) Acta Med. Scand. 5–103. [PubMed]
- 50.Old L J. Science. 1985;230:630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- 51.Mach N, Dranoff G. Curr Opin Immunol. 2000;12:571–575. doi: 10.1016/s0952-7915(00)00144-8. [DOI] [PubMed] [Google Scholar]
- 52.Colombo M P, Ferrari G, Stoppacciaro A, Parenza M, Rodolfo M, Mavillo F, Parmiani G. J Exp Med. 1991;173:889–897. doi: 10.1084/jem.173.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khattri R, Auger J A, Griffin M D, Sharpe A H, Bluestone J A. J Immunol. 1999;162:5784–5791. [PubMed] [Google Scholar]
- 54.Hodi F S, Schmollinger J C, Soiffer R J, Salgia R, Lynch T, Ritz J, Alyea E P, Yang J C, Neuberg D, Mihm M, Dranoff G. Proc Natl Acad Sci USA. 2002;99:6919–6924. doi: 10.1073/pnas.102025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Metz D P, Farber D L, Taylor T, Bottomly K. J Immunol. 1998;161:5855–5861. [PubMed] [Google Scholar]
- 56.Chambers C A, Kuhns M S, Allison J P. Proc Natl Acad Sci USA. 1999;96:8603–8608. doi: 10.1073/pnas.96.15.8603. [DOI] [PMC free article] [PubMed] [Google Scholar]