Abstract
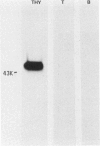
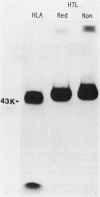
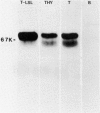
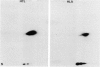
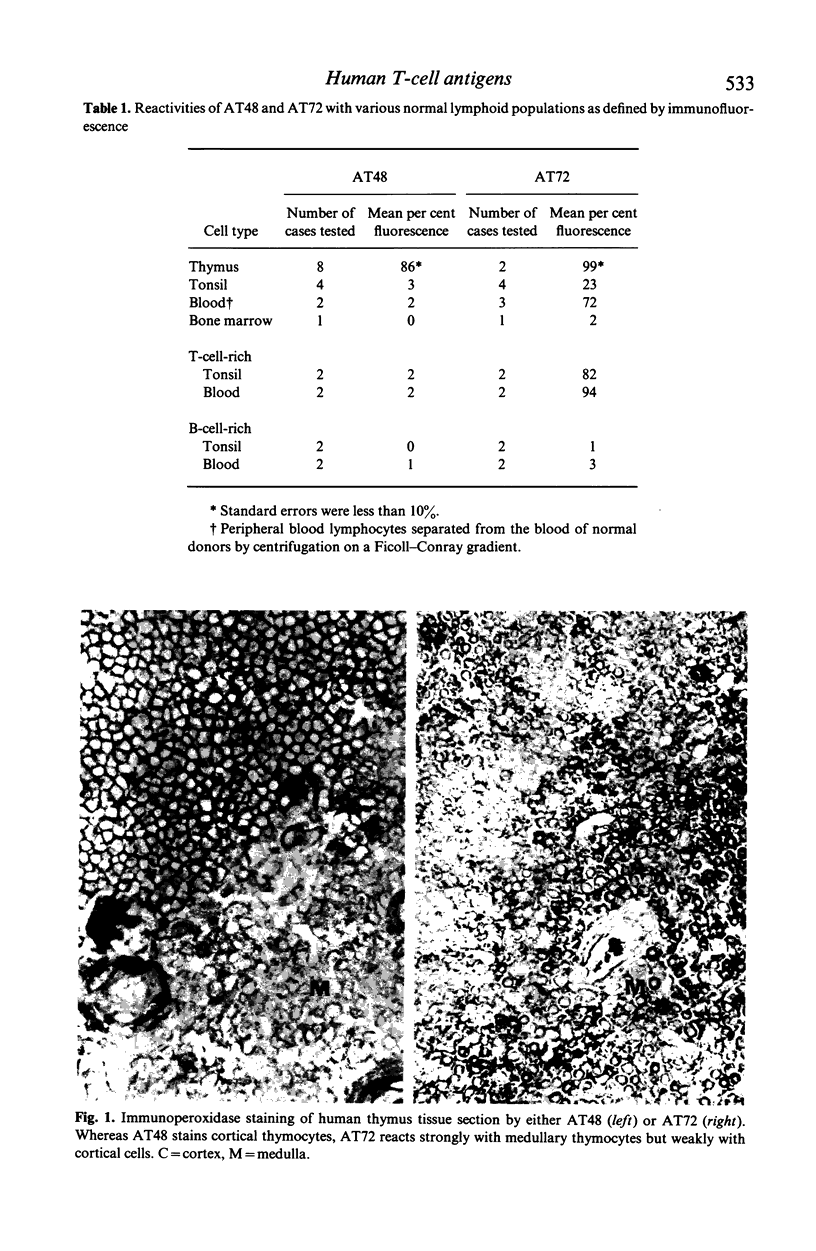
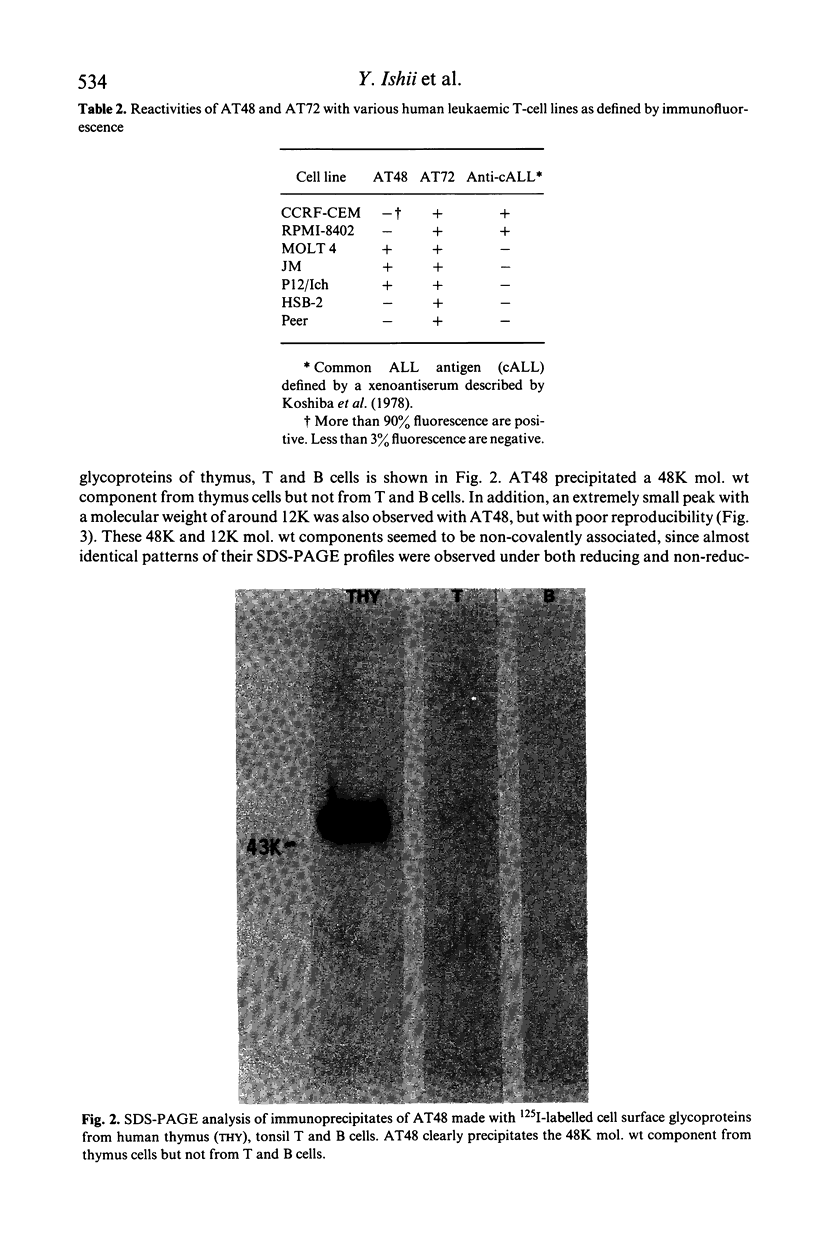
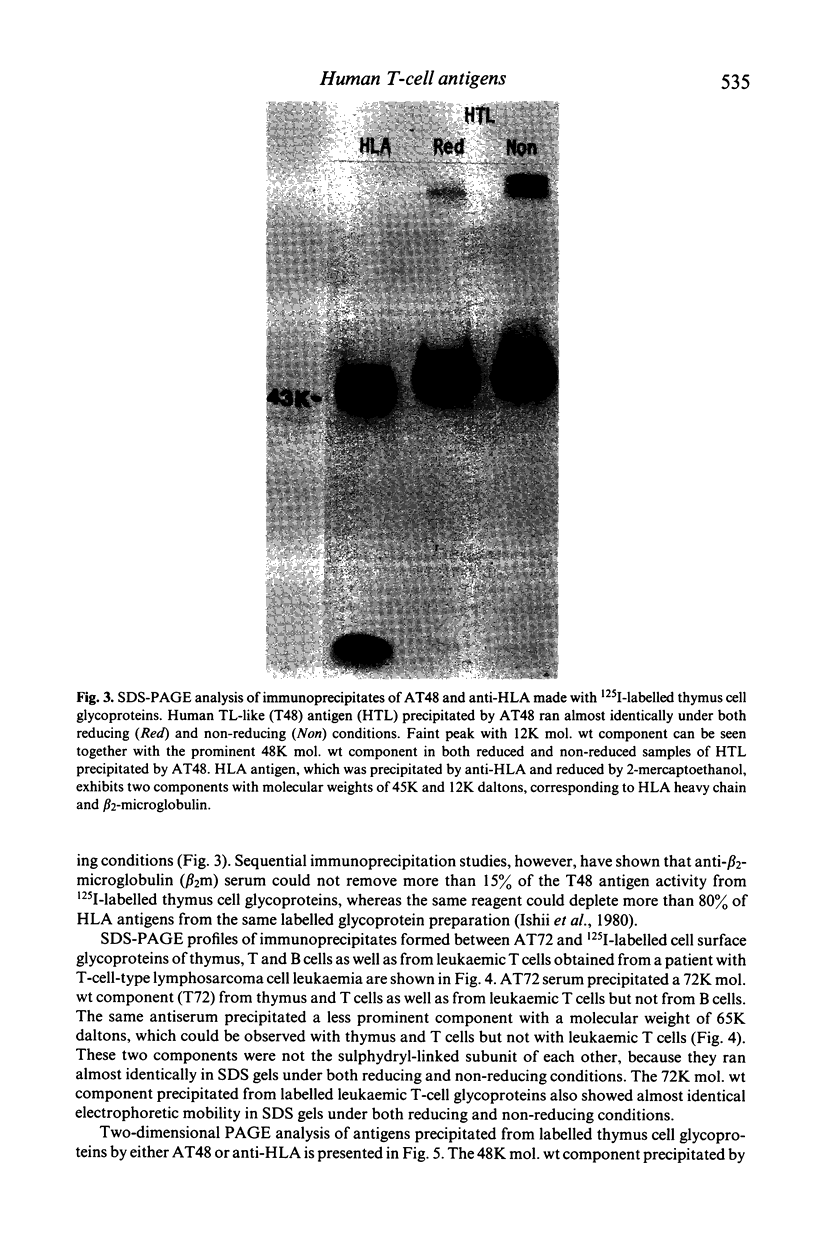
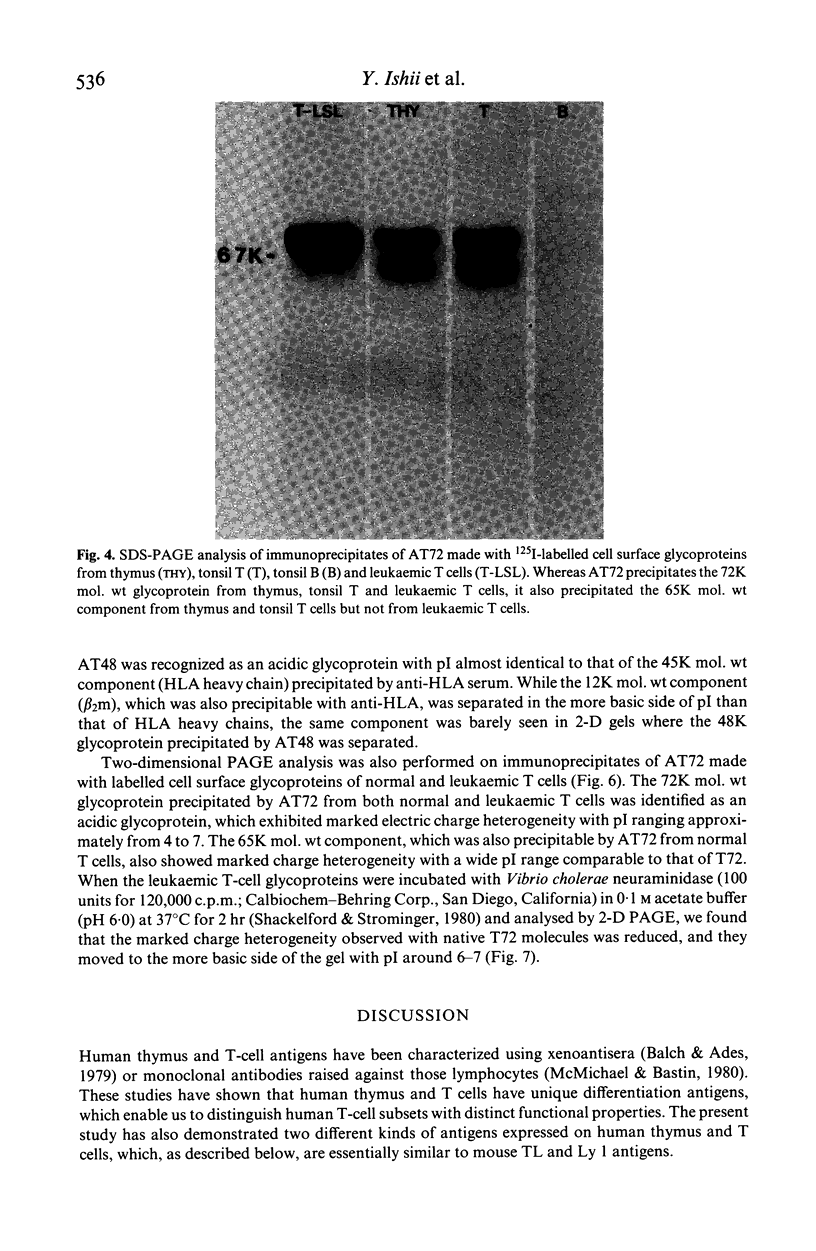
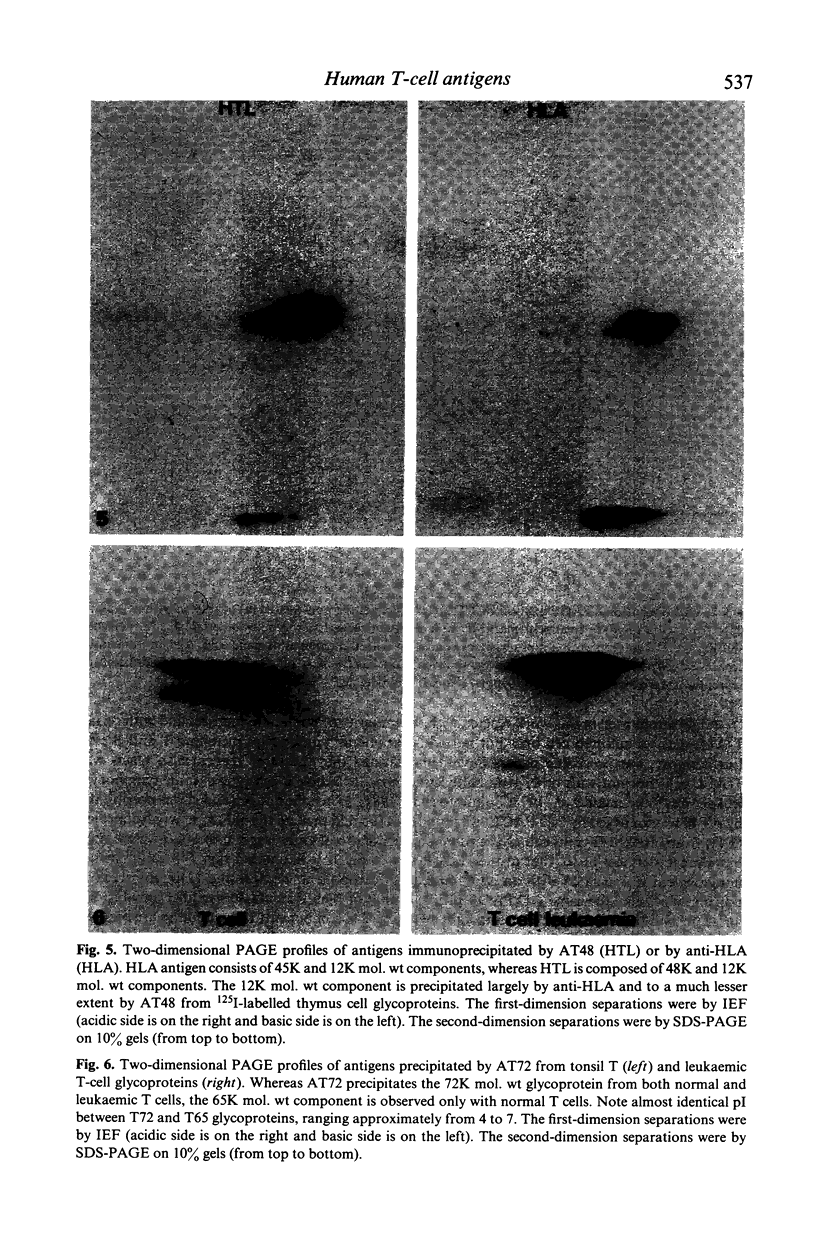
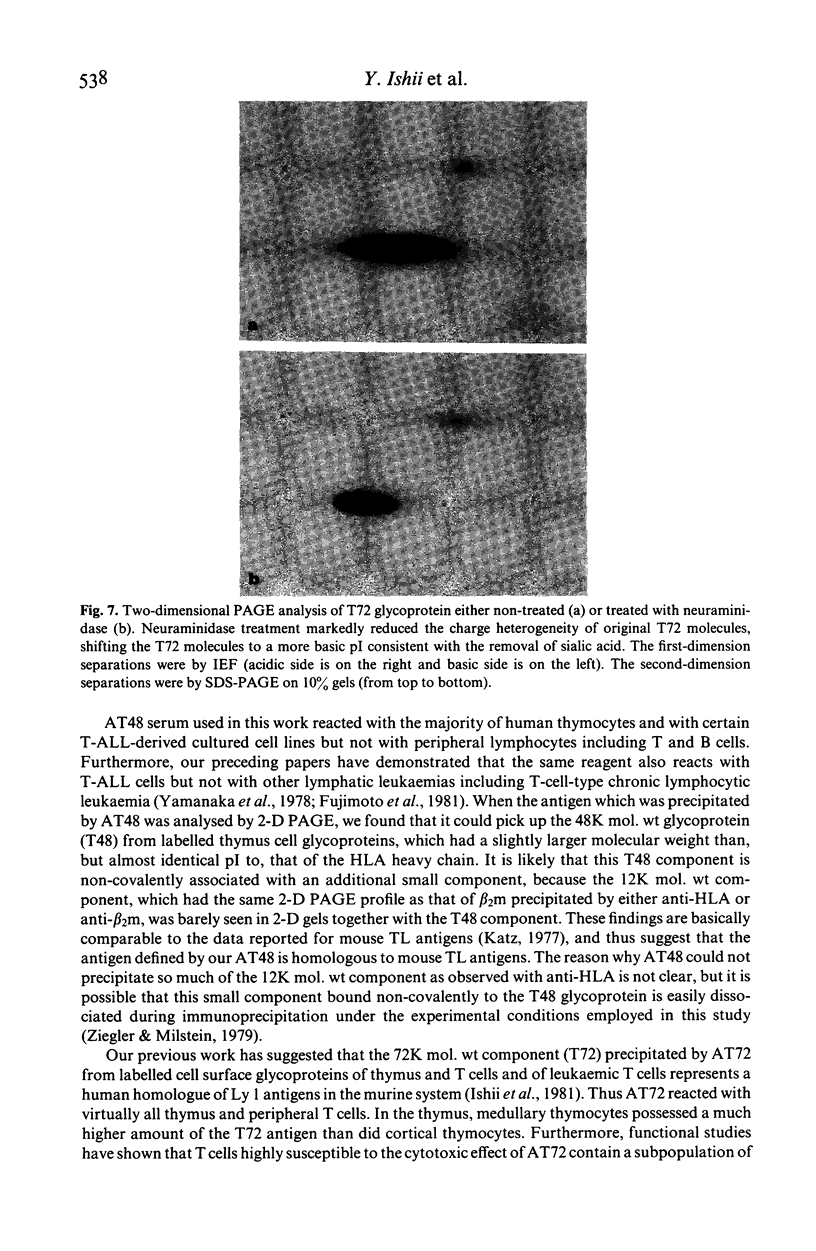
Xenoantisera, designated AT48 and AT72, were developed by immunizing rabbits with human thymus cell membrane and guinea-pigs with a T-cell glycoprotein purified from leukaemic T-cell membrane. Whereas AT48, after appropriate absorption, reacted exclusively with the majority of thymocytes (mainly cortical thymocytes) among normal lymphoid populations, AT72 reacted with virtually all of the thymus and T cells but not with B cells. Thymocytes, which were strongly reactive with AT72, existed in the thymic medulla, but cortical cells were also very weakly reactive with AT72. When cultured T-cell lines, all of which were derived from patients with T-cell-type acute lymphatic leukaemias, were tested for their reactivities with AT48 and AT72 by immunofluorescence, we found that AT48 stained certain T-cell lines, whereas AT72 stained all of the T-cell lines tested so far. Immunochemical data showed that AT48 precipitated a 48K molecular weight (mol. wt) glycoprotein from 125I-labelled thymus cell surface glycoproteins, which appeared to be very weakly associated with a 12K mol. wt component. These 48K and 12K mol. wt components precipitated by AT48 showed almost identical isoelectric points (pI) to those of HLA heavy chain and beta 2-microglobulin respectively. AT72, on the other hand, precipitated a 72K mol. wt glycoprotein from thymus and T cells as well as from leukaemic T cells. A less prominent 65K mol. wt glycoprotein was also precipitated by AT72 from thymus and T cells but not from leukaemic T cells. These two components showed almost identical pI ranging approximately from 4 to 7, and this marked charge heterogeneity observed was reduced by neuraminidase treatment, suggesting that it reflects the heterogeneity in sialylation of this molecular species. We concluded from these data that AT48 and AT72 used in this work could detect human homologues of mouse TL and Ly 1 antigens respectively.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch C. M., Ades E. W. Heterogeneity of cell surface xenoantigens on human T lymphocytes. J Reticuloendothel Soc. 1979 Jun;25(6):635–651. [PubMed] [Google Scholar]
- Bhan A. K., Reinherz E. L., Poppema S., McCluskey R. T., Schlossman S. F. Location of T cell and major histocompatibility complex antigens in the human thymus. J Exp Med. 1980 Oct 1;152(4):771–782. doi: 10.1084/jem.152.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradstock K. F., Janossy G., Pizzolo G., Hoffbrand A. V., McMichael A., Pilch J. R., Milstein C., Beverley P., Bollum F. J. Subpopulations of normal and leukemic human thymocytes: an analysis with the use of monoclonal antibodies. J Natl Cancer Inst. 1980 Jul;65(1):33–42. [PubMed] [Google Scholar]
- Cullen S. E., Schwartz B. D. An improved method for isolation of H-2 and Ia alloantigens with immunoprecipitation induced by protein A-bearing staphylococci. J Immunol. 1976 Jul;117(1):136–142. [PubMed] [Google Scholar]
- Fujimoto J., Ishii Y., Koshiba H., Matsuura A., Ogasawara M., Uede T., Kikuchi K. Human thymus antigen: characterization and its expression on human leukemias. Am J Hematol. 1981;10(2):145–156. doi: 10.1002/ajh.2830100206. [DOI] [PubMed] [Google Scholar]
- Hayman M. J., Crumpton M. J. Isolation of glycoproteins from pig lymphocyte plasma membrane using Lens culinaris phytohemagglutinin. Biochem Biophys Res Commun. 1972 May 26;47(4):923–930. doi: 10.1016/0006-291x(72)90581-5. [DOI] [PubMed] [Google Scholar]
- Ishii Y., Fujimoto J., Koshiba H., Kikuchi K. Isolation and partial characterization of a 72,000-dalton glycoprotein (Tgp72) on human thymus and T cells: possible relationship to mouse Ly-1 antigens. J Immunol. 1981 Jun;126(6):2171–2176. [PubMed] [Google Scholar]
- Ishii Y., Ueno H., Kikuchi K. Ultrastructure of lymphoid cells with surface-bound immunoglobulin in rats. J Reticuloendothel Soc. 1974 Feb;15(2):155–162. [PubMed] [Google Scholar]
- Koshiba H., Minowada J., Pressman D. Rabbit antiserum against a non-T, non-B leukemia cell line that carries the Ph1 chromosome (NALM-1): antibody specific to a non-T, non-B acute lymphoblastic leukemia antigen. J Natl Cancer Inst. 1978 Oct;61(4):987–991. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lay W. H., Mendes N. F., Bianco C., Nussenzweig V. Binding of sheep red blood cells to a large population of human lymphocytes. Nature. 1971 Apr 23;230(5295):531–532. doi: 10.1038/230531a0. [DOI] [PubMed] [Google Scholar]
- McMichael A. J., Pilch J. R., Galfré G., Mason D. Y., Fabre J. W., Milstein C. A human thymocyte antigen defined by a hybrid myeloma monoclonal antibody. Eur J Immunol. 1979 Mar;9(3):205–210. doi: 10.1002/eji.1830090307. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ravid Z., Goldblum N., Zaizov R., Schlesinger M., Kertes T., Minowada J., Verbi W., Greaves M. Establishment and characterization of a new leukaemic T-cell line (Peer) with an unusual phenotype. Int J Cancer. 1980 Jun 15;25(6):705–710. doi: 10.1002/ijc.2910250604. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford D. A., Strominger J. L. Demonstration of structural polymorphism among HLA-DR light chains by two-dimensional gel electrophoresis. J Exp Med. 1980 Jan 1;151(1):144–165. doi: 10.1084/jem.151.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. D., Nossal G. J. Identification of human T and B lymphocytes in normal peripheral blood and in chronic lymphocytic leukaemia. Lancet. 1971 Oct 9;2(7728):788–791. doi: 10.1016/s0140-6736(71)92741-3. [DOI] [PubMed] [Google Scholar]
- Ziegler A., Milstein C. A small polypeptide different from beta2-microglobin associated with a human cell surface antigen. Nature. 1979 May 17;279(5710):243–244. doi: 10.1038/279243a0. [DOI] [PubMed] [Google Scholar]