Abstract
The capture of photons by the photosynthetic apparatus is the first step in photosynthesis in all autotrophic higher plants. This light capture is dominated by pigment-containing proteins known as light-harvesting complexes (LHCs). The xanthophyll–carotenoid complement of these LHCs (neoxanthin, violaxanthin, and lutein) is highly conserved, with no deletions and few, uncommon additions. We report that neoxanthin, considered an integral component of LHCs, is stoichiometrically replaced by lutein-5,6-epoxide in the parasitic angiosperm Cuscuta reflexa, without compromising the structural integrity of the LHCs. Lutein-5,6-epoxide differs from neoxanthin in that it is involved in a light-driven deepoxidation cycle similar to the deepoxidation of violaxanthin in the xanthophyll cycle, which is implicated in protection against photodamage. The absence of neoxanthin and its replacement by lutein-5,6-epoxide changes our understanding of the structure-function relationship in LHCs, has implications for biosynthetic pathways involving neoxanthin (such as the plant hormone abscisic acid), and identifies one of the early steps associated with the evolution of heterotrophy from autotrophy in plants.
Despite the enormous diversity in the evolution, form, and function of photosynthetically active higher plants, the basic biochemical mechanisms appear remarkably conserved. In all autotrophic plants studied, the first step in photosynthesis, the absorption of sunlight, is carried out primarily by an array of pigment-binding proteins termed the light-harvesting complexes (LHCs). With no deletions and few, uncommon additions, these complexes contain the same major xanthophyll—carotenoids, namely lutein, neoxanthin, and violaxanthin. Of these xanthophylls, lutein appears in the highest concentration, usually comprising between 30 and 60% of total xanthophylls (1, 2). Studies elucidating the structure of the major pigment-binding protein of the LHCs of photosystem II (LHCIIb) suggest that each monomeric subunit contains two lutein molecules that form a crossbrace in the center of the complex indicating that lutein may have a major structural role within the LHCs (3, 4). Neoxanthin comprises a smaller proportion of the total xanthophyll pool but epitomizes the conservative nature of the photosynthetic apparatus in that its concentration is remarkably consistent across a wide range of contrasting species and growth environments, where it accounts for between 9 and 14% of total xanthophylls (1, 2). This apparent stoichiometry has led to the suggestion that neoxanthin, like lutein, is also an integral structural component of LHCs. Neoxanthin is also considered to be a precursor of abscisic acid (ABA) (5), a plant hormone thought to have multiple functions in higher plants including, among others, roles in the regulation of stomatal function and seed dormancy. Violaxanthin is the biosynthetic precursor to neoxanthin but differs from both lutein and neoxanthin in that its concentration varies markedly between species and depending on the light environment under which the plants have developed. Violaxanthin is also the primary substrate in a reversible light-driven deepoxidation reaction known as the xanthophyll cycle. In this cycle, violaxanthin is converted to zeaxanthin by means of the intermediate antheraxanthin under conditions where light energy absorbed by chlorophyll is in excess of that used in photosynthetic processes, and this conversion is reversed when light is no longer excessive. The accumulation of the deepoxidation products antheraxanthin and zeaxanthin in the photosynthetic apparatus is associated with harmless energy dissipation in the form of heat. The xanthophyll cycle is ubiquitous in higher plants and is implicated in protection against photodamage (6, 7).
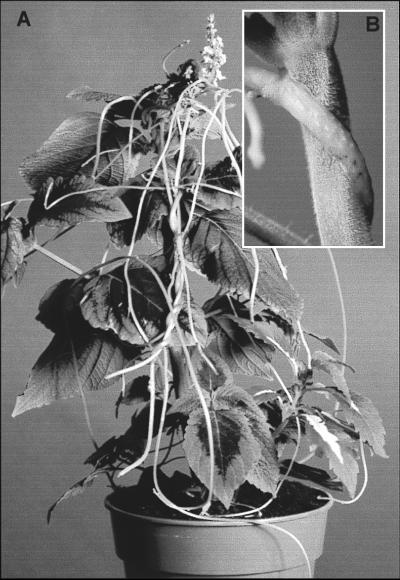
The Cuscutaceae is a monotypic family of ca. 150 parasitic plant species with low host specificity and a wide geographic distribution. Cuscuta is at an early stage of nonphotosynthetic evolution and the genus contains both chlorophyllous and achlorophyllous species, with the latter having been derived recently from the former (8–10). Thus Cuscuta provide a rare opportunity to observe the early evolutionary steps associated with the loss of photosynthesis and potential modifications to the otherwise widespread and highly conserved photosynthetic apparatus. The focus of this study is the chlorophyllous species C. reflexa (Fig. 1A), which contains just a single band of fully functional photosynthetic cells located adjacent to the vascular bundles (11). The functional significance of these cells is in the recycling of CO2 from neighboring cells that have high respiration rates. Fixation of external CO2 is unlikely because of spatial isolation from the atmosphere, and the parasite obtains carbon exclusively from the host plant, mainly by means of phloem connections in the haustoria (Fig. 1B), which provide a vascular bridge between the two species (12).
Figure 1.
Cuscuta species are parasitic on the above-ground parts of other plants. The parasites consist of a twining stem that lacks leaves and has no roots. They are dependent on the host plant for organic and inorganic solutes that they extract by means of haustoria, primarily from the host phloem (12). (A) C. reflexa growing on a Coleus host and (B) a close view of the host–parasite attachment with C. reflexa attached by haustoria to the stem of Coleus.
MATERIALS AND METHODS
Plant Material and Experimental Conditions.
Cuscuta reflexa Roxb. were grown with either Coleus blumei L. or Ricinus communis L. as the host species. Host plants and Spinacia oleracea (spinach) were grown in peat-based potting mix in a glasshouse with temperature in the range 15°C to 35°C under natural light conditions between April and September.
The pigment composition data presented in Fig. 2 and Table 1 were obtained from either plant material that had been maintained in the dark for a total of 12 h before sampling (dark-adapted) or plant material that had been dark-adapted and subsequently exposed to irradiance at 1,200 μmol m−2 s−1 for 2 to 4 h (light). The results in Fig. 3A are from a time-course analysis of the pigment composition after exposure of C. reflexa to high irradiance. In this experiment, C. reflexa were dark-adapted for 12 h before an initial time-zero sample was taken. Detached filaments were then exposed to irradiance of 1,200 μmol m−2 s−1 and light-exposed samples taken accordingly. At each sampling time, samples were plunged into liquid nitrogen where they were stored until HPLC analysis of pigments was carried out. The results in Fig. 3B are from a time-course analysis of carotenoid composition over a diurnal cycle for C. reflexa under normal growing conditions. In this experiment, samples for HPLC analysis of carotenoids were taken just before sunrise at midday and then just before sunrise the following day. Absorbance and 77 K fluorescence spectroscopy and sucrose centrifugation data presented in Fig. 4 are from thylakoids and LHCIIb purified from C. reflexa or S. oleracea immediately after either dark-adapted for 12 h or dark-adapted for 12 h followed by exposure to irradiance of 1,200 μmol m−2 s−1 for 3 h.
Figure 2.
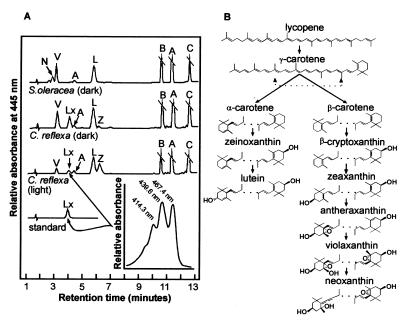
Carotenoid composition of higher plants. (A) HPLC separation of pigments from dark-adapted samples from S. oleracea (dark) and C. reflexa (dark), C. reflexa exposed to light at 1,200 μmol photons m−2 s−1 for 2–4 h [C. reflexa (light)]. The identification of the lutein-5,6-epoxide in C. reflexa was achieved by comparing its elution times and absorption spectrum (inset) with a pure sample of lutein-5,6-epoxide (standard); they were identical. N, neoxanthin; V, violaxanthin; Lx, lutein-5,6-epoxide; A, antheraxanthin; L, lutein; Z, zeaxanthin; a, chlorophyll a; b, chlorophyll b; C, β-carotene. (B) Biosynthetic pathway of carotenoids showing that neoxanthin is synthesized later than β-carotene-derived xanthophyll cycle carotenoids and distinct from α-carotene-derived lutein.
Table 1.
Pigment composition of Cuscuta reflexa and Spinacia oleracea
| % of total xanthophylls
|
||||||||
|---|---|---|---|---|---|---|---|---|
| N | V | Lx | A | L | Z | V + A + Z | a:b ratio | |
| C. reflexa | ||||||||
| Whole plant D | 0 | 28.6 ± 0.2 | 23.6 ± 1.8 | 2.0 ± 1.3 | 35.9 ± 1.6 | 0 | 41.0 ± 10 | 3.63 ± 0.20 |
| ThylakoidsD | 0 | 31.0 ± 2.0 | 22.8 ± 1.5 | 3.2 ± 0.8 | 43.9 ± 2.0 | 0 | 31.0 ± 2.0 | 3.39 ± 0.06 |
| LHCllbD | 0 | 8.0 ± 5.0 | 24.0 ± 1.5 | 0 | 68.3 ± 3.0 | 0 | 8.0 ± 5.0 | 1.34 ± 0.03 |
| LHCllbLi | 0 | 6.0 ± 0.6 | 16.0 ± 1.0 | 3.3 ± 0.6 | 66.5 ± 1.2 | 7.6 ± 1.3 | 16.9 ± 1.6 | 1.39 ± 0.02 |
| S. oleracea | ||||||||
| ThylakoidsD | 12.2 ± 0.1 | 31.5 ± 1.5 | 0 | 3.0 ± 0.5 | 53.3 ± 2.0 | 0 | 34.6 ± 1.5 | 3.46 ± 0.05 |
| LHCllbD | 24.2 ± 1.0 | 5.6 ± 4.0 | 0 | 0 | 70.0 ± 3.0 | 0 | 5.6 ± 4.0 | 1.32 ± 0.03 |
D, samples from plant material that had been dark-adapted for 12 h; Li, Samples from plant material after exposure to light at 1,200 μmol m−2 s−1 for 2 h. Pigments are identified as in Fig. 2. Errors are ±1 s.e.
Figure 3.
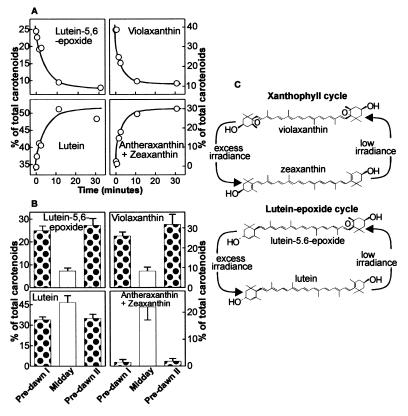
Light-driven carotenoid cycles in C. reflexa. (A) Time-course analysis of xanthophylls after exposure of C. reflexa filaments to high irradiance. Plants were dark adapted for 12 h before exposure to 1,200 μmol m−2 s−1. (B) Analysis of xanthophylls from C. reflexa over a diurnal period. Samples were taken predawn (predawn I), midday, and predawn the following day (predawn II). We propose that two distinct light-driven carotenoid cycles operate in C. reflexa (C): the xanthophyll cycle and a lutein-5,6-epoxide cycle.
Figure 4.
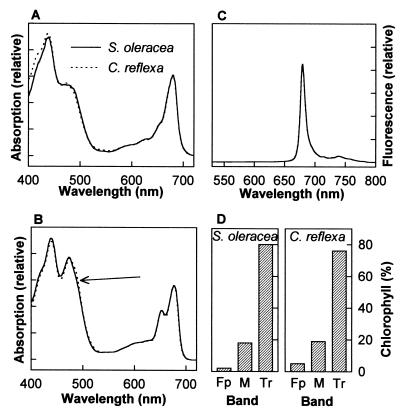
Lutein-5,6-epoxide-containing and neoxanthin-containing thylakoids and LHCIIb. The absorption spectra of C. reflexa and S. oleracea thylakoids (A) and LHCIIb (B). The arrow indicates the accentuated absorption at around 490 nm. (C) The 77 K fluorescence spectra of LHCIIb from C. reflexa and S. oleracea (overlapping). (D) Similar preparations of LHCIIb followed by centrifugation through a sucrose concentration gradient to separate bands of trimers (Tr) from monomers (M) of LHCIIb and free pigment (Fp).
Pigment Determination.
The pigment composition of C. reflexa was determined by HPLC by using a Waters Nova-Pak C18 radial compression column as described ref. 2. Whole-plant samples were frozen in liquid N2 immediately after sampling. Pigments were extracted from samples under low light by grinding in liquid N2 followed by grinding in degassed 100% acetone at 0–4°C. On occasion a small amount of NaHCO3 (around 0.1 g⋅g−1 plant material) was added to plant material before extraction to ensure no acidification of the extract occurred; however, this had no influence on carotenoid composition. Pigments were extracted from thylakoids and isoelectric focusing protein bands by adding 100% acetone to samples to give a final concentration of 90% acetone. Lutein-5,6-epoxide ([3S, 5R, 6S, 3′R, 6′R]-5, 6-epoxy-5, 6-dihydro-β,ɛ-carotene-3, 3′-diol; taraxanthin) in C. reflexa was identified by comparing its elution times and absorption spectrum with a pure sample. All other pigments were identified as described (2).
Separation and Purification of Thylakoids and LHCs.
Thylakoids from C. reflexa were prepared by grinding plant material in a polytron tissue homogenizer in pH 6.5 buffer (100 mmol l−1 Hepes/0.3 mol l−1 sorbitol) containing 2% (wt/vol) polyvinylpyrrolidone/0.5% (wt/vol) BSA/0.5 mmol l−1 EDTA. Homogenate was then filtered twice through four layers of cheesecloth. The filtered solution was centrifuged (5,000 g for 10 min), and the resulting pellet was resuspended, first in pH 7.3 buffer, then pH 7.6 buffer containing 1.0 mmol l−1 EDTA. The final thylakoid pellet was resuspended in pure water. Thylakoids of S. oleracea were prepared as described (13). LHCs were separated by isoelectric focusing and protein bands identified as described (13). LHCIIb is thought to exist in the thylakoid membrane primarily in a trimeric state (4, 14). To determine the proportion of LHCIIb in a trimeric state and therefore the stability of trimers, LHCIIb from C. reflexa and S. oleracea were subject to centrifugation through a sucrose gradient as described (15).
Absorbance and Fluorescence Spectroscopy.
Absorbance measurements of thylakoids and LHCIIb from C. reflexa and S. oleracea were carried out at room temperature by using an Aminco DW2000 dual wavelength spectrophotometer as described (16). Low-temperature fluorescence of LHCIIb from C. reflexa and S. oleracea was measured at 77 K by using a liquid nitrogen cryostat and an excitation wavelength of 435 nm. Fluorescence spectra were detected by using a silicon photodiode detector as described (16).
RESULTS AND DISCUSSION
The photosynthetic pigment composition of C. reflexa contrasts markedly with that of the model plant species, S. oleracea (Fig. 2B). Like all unmodified photosynthetically active higher plants studied to date, S. oleracea contains chlorophylls a and b, the carotenoid β-carotene, and the xanthophyll–carotenoids neoxanthin, violaxanthin, and lutein. The presence of neoxanthin has been reported in all higher plants studied regardless of lifeform, habitat, or phylogeny (1, 17–19). C. reflexa does not contain neoxanthin, and instead has substantial amounts of another xanthophyll, identified as lutein-5,6-epoxide (Fig. 2A; Table 1). To our knowledge, this is the first report of a naturally occurring photosynthetically active higher plant that does not contain neoxanthin. The only other evidence to support neoxanthin substitution is derived from a small number of recent studies on mutant plants, where missing xanthophylls were substituted by other carotenoids commonly present in the LHCs (20, 21). Moreover, unlike in C. reflexa, these studies also show slight differences in the partitioning of chlorophyll to LHCIIb.
Neoxanthin is formed from violaxanthin, which originates from the epoxidation of zeaxanthin, which is in turn derived from β-carotene (Fig. 2B). Violaxanthin is present in C. reflexa, indicating that the plant lacks the synthesis step only from violaxanthin to neoxanthin. Violaxanthin is not only the biosynthetic precursor to neoxanthin (Fig. 2B) but is also the primary substrate in a reversible light-driven deepoxidation reaction known as the xanthophyll cycle. In this cycle, violaxanthin is converted to zeaxanthin by means of the intermediate antheraxanthin under conditions where light energy absorbed by chlorophyll is in excess of that used in photosynthesis and is reversed when light is no longer excessive. Such photoprotective mechanisms are essential for oxygen-producing plants and principally result from the accumulation of xanthophyll cycle products (6, 7, 22). Carotenoid composition in C. reflexa was determined before and after exposure to high irradiance to determine whether the xanthophyll cycle was operational and to determine whether irradiance altered the amount of lutein-5,6-epoxide (Fig. 2A). Deepoxidation of the xanthophyll cycle pigments was clearly evident. Plants exposed to high irradiance had lower amounts of violaxanthin and greater amount of its deepoxidation products, antheraxanthin and particularly zeaxanthin, compared with dark-adapted plants. Lower concentrations of lutein-5,6-epoxide were also evident in irradiated plants. The disappearance of lutein-5,6-epoxide was as rapid as that of violaxanthin after exposure to high irradiance and over 60% of the pigment was lost after a 10-min period of irradiation (Fig. 3A). The decrease in the concentration of lutein-5,6-epoxide was matched by a concomitant increase in lutein, just as the loss of violaxanthin was matched by the appearance of its deepoxidation products, antheraxanthin and zeaxanthin. No increases in the concentration of lutein were observed after exposure of spinach to high light (data not shown). These data suggest that in C. reflexa, lutein-5,6-epoxide, like violaxanthin, is subject to deepoxidation on exposure to high irradiance.
In higher plants grown under natural light, the xanthophyll cycle typically shows a distinct diel pattern with a low deepoxidation state predawn and a high deepoxidation at midday (23), presumably reflecting the requirement for energy dissipation depending on light intensity. C. reflexa not only showed a diel pattern characteristic of the xanthophyll cycle involving violaxanthin, but also a similar diel pattern in the amounts of lutein-5,6-epoxide and lutein (Fig. 3B). Thus, we propose the existence of a second carotenoid cycle in C. reflexa that, like the xanthophyll cycle, involves the rapid reversible light-driven deepoxidation of lutein-5,6-epoxide to lutein (Fig. 3C). This deepoxidation increases the number of conjugated carbon double bonds from 10 to 11, with a subsequent influence on polarity (as evidenced by greater retention time during reversed-phase HPLC, Fig. 2A). Increases in the number of conjugated carbon double bonds and changes in polarity and end-group orientation are also central to proposed photoprotection mechanisms by the deepoxidation of violaxanthin (7, 24), indicative of a similar role in C. reflexa for the lutein–epoxide cycle. Interestingly, recent work on mutants of the green algae chlamydomonas does show that lutein, like the deepoxidation products of violaxanthin, may have a significant role in energy dissipation (21, 25).
Apart from the replacement of neoxanthin by lutein-5,6-epoxide, thylakoids of C. reflexa were almost identical to those of S. oleracea. Both had similar chlorophyll a:b ratios and similar concentrations of lutein and xanthophyll cycle pigments (Table 1). The absorption spectra of thylakoids were also nearly identical (Fig. 4A). These results demonstrate that lutein-5,6-epoxide effectively replaces neoxanthin in the LHCs of C. reflexa without significantly influencing thylakoid structure. Within thylakoids, the majority of neoxanthin is located within the major light-harvesting complex, LHCIIb, which usually binds more than 40% of total chlorophyll and is considered to have one molecule of neoxanthin bound to each polypeptide (14). In LHCIIb purified from C. reflexa thylakoids, lutein-5,6-epoxide accounted for 24% of the bound carotenoids, the same as the proportion of neoxanthin in LHCIIb from S. oleracea (Table 1). Purification of the LHCIIb from C. reflexa and S. oleracea did not result in significant loss of lutein-5,6-epoxide or neoxanthin, respectively, indicating that both xanthophylls were tightly bound to the pigment–protein complex. Again, like thylakoids, the LHCIIb in the two species were almost indistinguishable in other respects: chlorophyll a:b ratios and lutein composition were the same (Table 1). The absorbance and 77 K fluorescence spectra of LHCIIb were also remarkably similar (Fig. 4 B and C). The absorption spectra reflect the combined absorption of chlorophylls and carotenoids within the complex. The only feature distinguishing the absorption spectra of thylakoids of C. reflexa from those of S. oleracea was an accentuated absorption at around 490 nm. At these wavelengths, absorbance is caused primarily by carotenoids and most likely reflects the substitution of neoxanthin by lutein-5,6-epoxide. In contrast to absorption, the 77 K fluorescence spectra is a result of fluorescence that is primarily from chlorophylls and not carotenoids. The identical 77 K fluorescence spectra indicate that the organization of chlorophyll populations and the energy transfer between pigments are not altered by replacement of neoxanthin by lutein-5,6-epoxide (16). Finally, the similarity between the lutein-5,6-epoxide-containing LHCIIb from C. reflexa and the neoxanthin-containing LHCIIb from S. oleracea is reflected by the stability of LHCIIb trimers. In thylakoids, LHCIIb is thought to exist primarily in a trimeric state (4, 14). Sucrose centrifugation showed that this was the case for both C. reflexa and S. oleracea (Fig. 4D). Neoxanthin was presumed to be a ubiquitous component of LHCs; however, our results clearly show that this xanthophyll is replaced in C. reflexa by lutein-5,6-epoxide without compromising the structure of LHCIIb, demonstrating that the binding and functional role of neoxanthin within the LHCs is not unique.
Analysis of the carotenoid composition of LHCIIb from C. reflexa that had been exposed to high irradiance contained, like whole plant and thylakoid samples, less lutein-5,6-epoxide than dark-adapted samples (Table 1), confirming that even this tightly bound lutein-5,6-epoxide within LHCIIb was subject to deepoxidation. Thus, this newly discovered carotenoid cycle is, like the xanthophyll cycle (13), operating on carotenoids bound to the LHCs. It is important to note that although lutein-5,6-epoxide replaces neoxanthin in the LHCIIb of C. reflexa, only the former is subject to deepoxidation, despite common structural end-groups (Figs. 2B and 3C). Violaxanthin deepoxidase, the enzyme responsible for the deepoxidation of violaxanthin and antheraxanthin in the xanthophyll cycle, has been isolated from plants and shown to be substrate specific. Although it can deepoxidize lutein-5,6-epoxide at similar rate to violaxanthin, deepoxidation of neoxanthin is limited (26). These results and the similar kinetics of deepoxidation observed in our experiments (Fig. 3A) suggest that violaxanthin deepoxidase may also be responsible for the deepoxidation of lutein-5,6-epoxide in C. reflexa. However, the low-irradiance reversal of this reaction (epoxidation) in the lutein-epoxide cycle (Fig. 3C) appears to be restricted to lutein initially derived from lutein-5,6-epoxide and does not include the two lutein molecules that are normally present within the LHCs. In the structural model for LHCIIb, these two luteins are suggested to be bound internally within the complex (3), thereby explaining their unavailability for epoxidation and suggesting that the lutein involved in the lutein–epoxide cycle is bound toward the periphery of the complex, presumably in the location normally occupied by neoxanthin.
The biosynthetic pathway of lutein-5,6-epoxide is unknown. However, as lutein is an α-carotene derived-carotenoid, it seems unlikely that lutein-5,6-epoxide is derived from the β-carotene branch of the biosynthetic pathway (Fig. 2B). Previous reports suggest that there is no net change in carotenoid synthesis in mutants disrupted in either branch of xanthophyll synthesis (27, 28), and it is possible that the accumulation of lutein-5,6-epoxide in C. reflexa reflects the compensatory synthesis to α-carotene-derived xanthophylls when production of the β-carotene-derived neoxanthin is limited. The absence of neoxanthin in C. reflexa may have wider implications for plant metabolism. Recent evidence suggests that it is a precursor of the plant growth regulator ABA (5, 29), and thus C. reflexa will be unable to produce ABA from this source. Parasitic angiosperms will also have access to host-derived sources of ABA, thus circumventing the requirement to synthesize ABA through the carotenoid pathway. Therefore the widespread conservation of neoxanthin among higher plants may reflect its role in other biosynthetic processes, specifically ABA synthesis, rather than a specific role within LHCs. In C. reflexa, it is possible that the provision of host ABA resulted in the redundancy of neoxanthin rather than loss or modification of photosynthetic apparatus because of a reliance on host carbon. Cuscuta species provide an opportunity to examine plants on the evolutionary watershed between autotrophy and heterotrophy. Our results demonstrate how the study of such species provide insights not only into processes of plant evolution but also into the more specific areas of biochemistry and biophysics.
Acknowledgments
We thank Andy J. Young, School of Biological and Earth Sciences, Liverpool John Moores University, Liverpool, U.K., for assistance in identifying lutein-5,6-epoxide, and Neil Hunter, Department of Molecular Biology and Biotechnology, University of Sheffield, U.K., for the use of HPLC equipment.
ABBREVIATIONS
- LHCs
light-harvesting complexes
- LHCIIb
the major pigment-binding protein of the LHCs of photosystem II
- ABA
abscisic acid
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Johnson G N, Young A J, Scholes J D, Horton P. Plant Cell Environ. 1993;16:673–679. [Google Scholar]
- 2.Bungard R A, McNeil D, Morton J D. Aust J Plant Physiol. 1997;24:205–214. [Google Scholar]
- 3.Kühlbrandt W, Wang D N, Fujiyoshi Y. Nature (London) 1994;367:614–621. doi: 10.1038/367614a0. [DOI] [PubMed] [Google Scholar]
- 4.Kühlbrandt W, Wang D N. Nature (London) 1991;350:130–134. doi: 10.1038/350130a0. [DOI] [PubMed] [Google Scholar]
- 5.Lee H-S, Milborrow B V. Aust J Plant Pysiol. 1997;24:715–726. [Google Scholar]
- 6.Demmig-Adams B. Biochim Biophys Acta. 1990;1020:1–24. [Google Scholar]
- 7.Horton P, Ruban A V, Walters R G. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- 8.Haberhausen G, Zetsche K. Plant Mol Biol. 1994;24:217–222. doi: 10.1007/BF00040588. [DOI] [PubMed] [Google Scholar]
- 9.dePamphilis C W. In: Parasitic Plants. Press M C, Graves J D, editors. London: Chapman & Hall; 1995. pp. 177–205. [Google Scholar]
- 10.Nickrent D L, Duff R J, Colwell A E, Wolfe A D, Young N D, Steiner K E, dePamphilis C W. In: Molecular Systematics of Plants II. DNA Sequencing. Soltis D E, Soltis P S, Doyle J J, editors. Boston: Kluwer; 1998. pp. 211–241. [Google Scholar]
- 11.Hibberd J M, Bungard R A, Press M C, Jeschke W D, Scholes J D, Quick W P. Planta. 1998;205:506–513. [Google Scholar]
- 12.Jeschke W D, Rath N, Baumel P, Czygan F C, Proksch P. J Exp Bot. 1994;45:791–800. [Google Scholar]
- 13.Ruban A V, Young A, Pascal A, Horton P. Plant Physiol. 1994;104:227–234. doi: 10.1104/pp.104.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peter G F, Thornber J P. J Biol Chem. 1991;226:16745–16754. [PubMed] [Google Scholar]
- 15.Ruban A V, Phillip D, Young A, Horton P. Biochemistry. 1997;36:7855–7859. doi: 10.1021/bi9630725. [DOI] [PubMed] [Google Scholar]
- 16.Ruban A V, Horton P. Biochim Biophys Acta. 1992;1102:30–38. [Google Scholar]
- 18.Demmig-Adams B, Adams W W. Plant Cell Environ. 1992;15:411–419. [Google Scholar]
- 19.Ruban A, Young A, Horton P. Plant Physiol. 1993;102:741–750. doi: 10.1104/pp.102.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strain H H. In: Biochemistry of Chloroplasts. Goodwin T W, editor. London: Academic; 1966. pp. 387–406. [Google Scholar]
- 20.Hurry V, Anderson J M, Chow W S, Osmond C B. Plant Physiol. 1997;113:639–648. doi: 10.1104/pp.113.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pogson B J, Niyogi K K, Björkman O, DellaPenna D. Proc Natl Acad Sci USA. 1998;95:13324–13329. doi: 10.1073/pnas.95.22.13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demmig-Adams B, Adams W W. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:599–626. [Google Scholar]
- 23.Adams W W, Demmig-Adams B. Planta. 1992;186:390–398. doi: 10.1007/BF00195320. [DOI] [PubMed] [Google Scholar]
- 24.Frank H A, Cua A, Chynwat V, Young A J, Goztola D, Wasielewski M R. Photosynth Res. 1994;41:389–395. doi: 10.1007/BF02183041. [DOI] [PubMed] [Google Scholar]
- 25.Niyogi K K, Björkman O, Grossman A R. Proc Natl Acad Sci USA. 1997;94:14162–14167. doi: 10.1073/pnas.94.25.14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto H Y, Higashi R M. Arch Biochem Biophys. 1978;190:514–522. doi: 10.1016/0003-9861(78)90305-3. [DOI] [PubMed] [Google Scholar]
- 27.Rock C D, Zeevaart J A D. Proc Natl Acad Sci USA. 1991;88:7496–7499. doi: 10.1073/pnas.88.17.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pogson B, McDonald K A, Troung M, Britton G, DellaPenna D. Plant Cell. 1996;8:1627–1639. doi: 10.1105/tpc.8.9.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartz S H, Tan B C, Gage D A, Zeevaart J A D, McCarty D R. Science. 1997;276:1872–1874. doi: 10.1126/science.276.5320.1872. [DOI] [PubMed] [Google Scholar]