Abstract
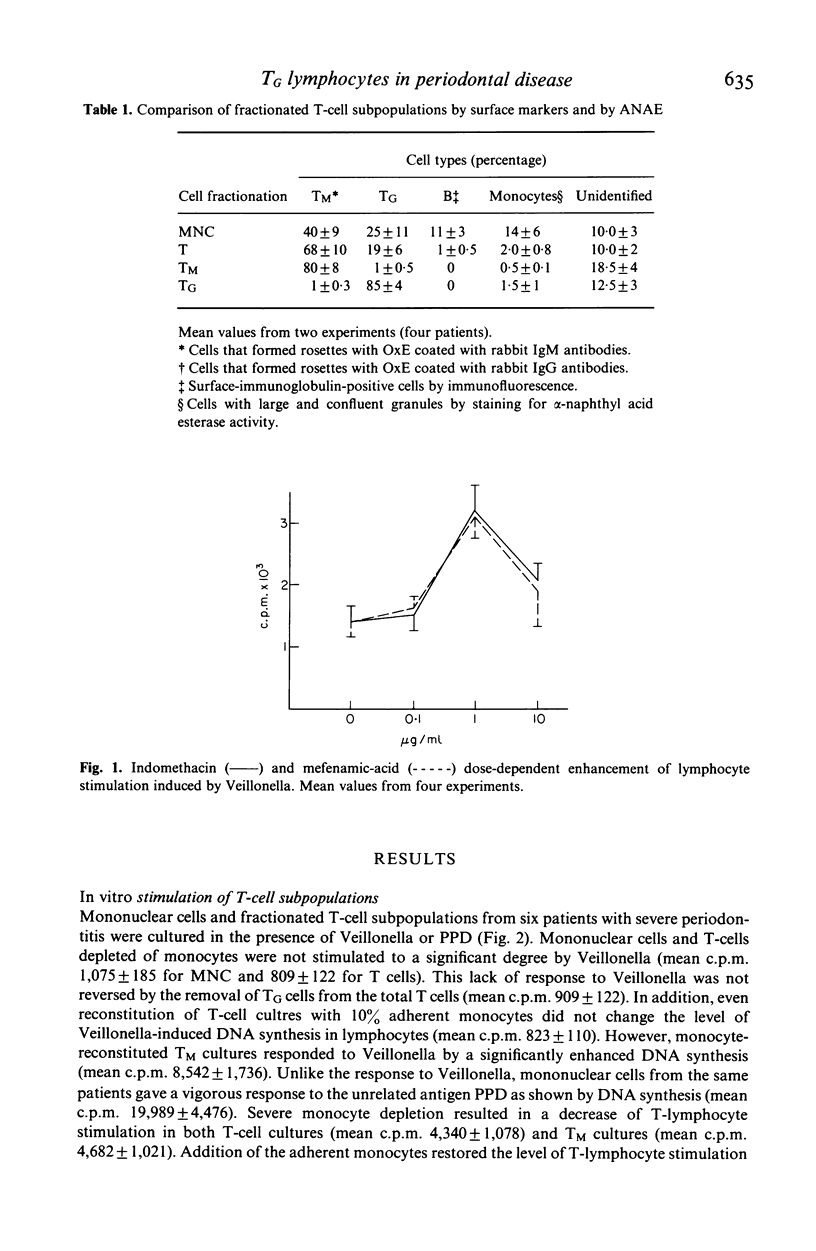
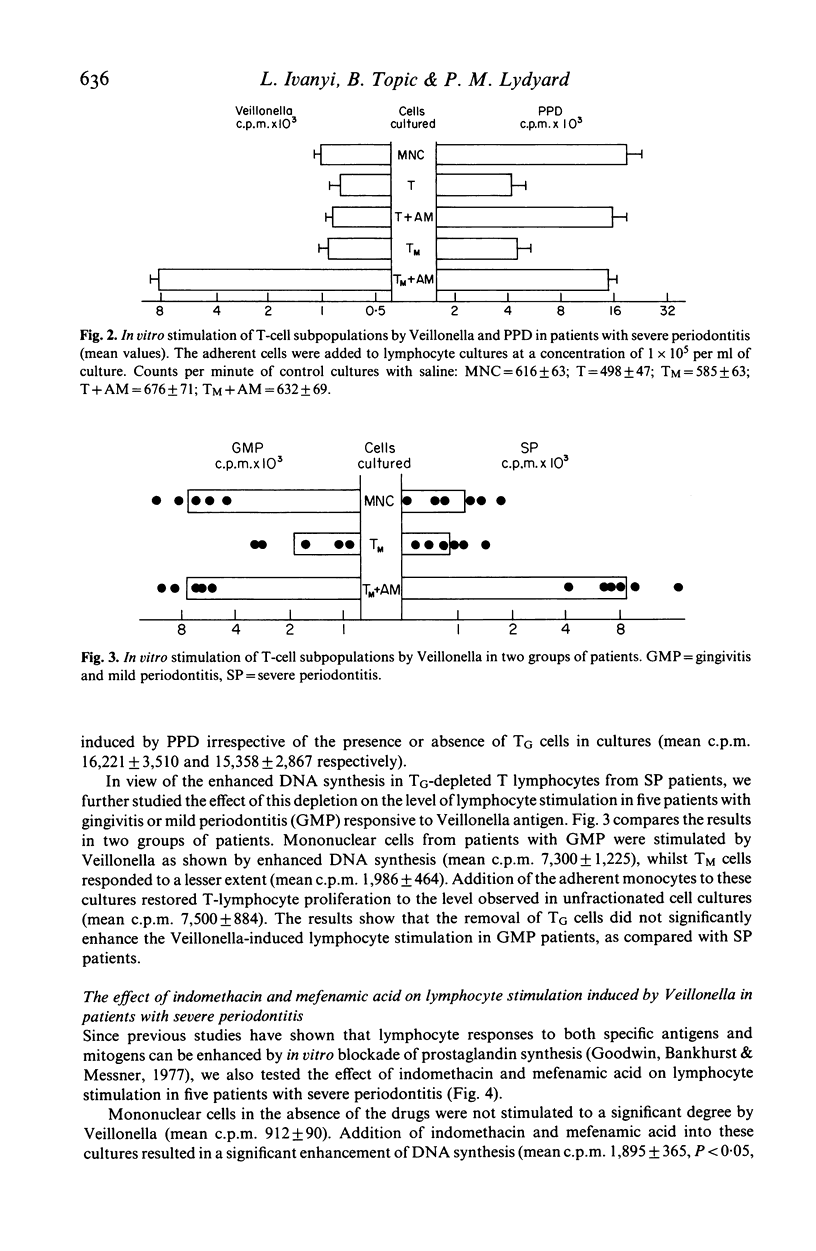
Blood mononuclear cell suspensions from patients with a severe form of periodontal disease failed to respond by in vitro stimulation to a sonicate from the oral bacterium, Veillonella alcalescens. The proliferative response could be restored by the depletion of TG cells by rosetting with IgG-coated ox erythrocytes and by reconstitution of the cell suspension with 10% plastic-adherent monocytes. Small but statistically significant restoration of the Veillonella response was also achieved by the addition of indomethacin or mefenamic acid to unfractionated cell cultures, indicating only a minor role of prostaglandin (PG) synthesis in the expression of suppressor cells. Since the in vitro response to an unrelated antigen PPD had been found unimpaired, the described TG-cell-mediated suppression of the Veillonella response is apparently antigen-specific.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker J. J., Wright W. E., Chan S. P., Oppenheim J. J. Longitudinal effects of clinical therapy and the edentulous state on the transformation of lymphocytes from patients with severe periodontitis. Clin Exp Immunol. 1978 Nov;34(2):199–205. [PMC free article] [PubMed] [Google Scholar]
- Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968 Sep;6(6):761–764. doi: 10.1097/00007890-196809000-00002. [DOI] [PubMed] [Google Scholar]
- Clagett J. A., Page R. C. Insoluble immune complexes and chronic periodontal diseases in man and the dog. Arch Oral Biol. 1978;23(3):153–165. doi: 10.1016/0003-9969(78)90211-x. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Bankhurst A. D., Messner R. P. Suppression of human T-cell mitogenesis by prostaglandin. Existence of a prostaglandin-producing suppressor cell. J Exp Med. 1977 Dec 1;146(6):1719–1734. doi: 10.1084/jem.146.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J. S., Kaszubowski P. A., Williams R. C., Jr Cyclic adenosine monophosphate response to prostaglandin E2 on subpopulations of human lymphocytes. J Exp Med. 1979 Nov 1;150(5):1260–1264. doi: 10.1084/jem.150.5.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J. S., Webb D. R. Regulation of the immune response by prostaglandins. Clin Immunol Immunopathol. 1980 Jan;15(1):106–122. doi: 10.1016/0090-1229(80)90024-0. [DOI] [PubMed] [Google Scholar]
- Ivanyi L., Challacombe S. J., Lehner T. The specificity of serum factors in lymphocyte transformation in periodontal disease. Clin Exp Immunol. 1973 Aug;14(4):491–500. [PMC free article] [PubMed] [Google Scholar]
- Ivanyi L., Lehner T. Interdependence of in vitro responsiveness of cord and maternal blood lymphocytes to antigens from oral bacteria. Clin Exp Immunol. 1977 Nov;30(2):252–258. [PMC free article] [PubMed] [Google Scholar]
- Ivanyi L., Lehner T. Stimulation of human lymphocytes by B-cell mitogens. Clin Exp Immunol. 1974 Nov;18(3):347–356. [PMC free article] [PubMed] [Google Scholar]
- Ivanyi L., Lehner T. Stimulation of lymphocyte transformation by bacterial antigens in patients with periodontal disease. Arch Oral Biol. 1970 Nov;15(11):1089–1096. doi: 10.1016/0003-9969(70)90121-4. [DOI] [PubMed] [Google Scholar]
- Katz P., Haynes B. F., Fauci A. S. Alteration of T-lymphocyte subpopulations in sarcoidosis. Clin Immunol Immunopathol. 1978 Jul;10(3):350–354. doi: 10.1016/0090-1229(78)90192-7. [DOI] [PubMed] [Google Scholar]
- Kemp J., Louie D., Mattingly J., Bennett J., Higuchi C., Pretell J., Horowitz M., Gershon R. Suppressor cells in vitro: differential effects of indomethacin and related compounds. J Immunopharmacol. 1980;2(4):471–489. doi: 10.3109/08923978009026407. [DOI] [PubMed] [Google Scholar]
- Kulenkampff J., Janossy G., Greaves M. F. Acid esterase in human lymphoid cells and leukaemic blasts: a marker for T lymphocytes. Br J Haematol. 1977 Jun;36(2):231–240. doi: 10.1111/j.1365-2141.1977.tb00644.x. [DOI] [PubMed] [Google Scholar]
- Listgarten M. A., Johnson D., Nowotny A., Tanner A. C., Socransky S. S. Histopathology of periodontal disease in gnotobiotic rats monoinfected with Eikenella corrodens. J Periodontal Res. 1978 Mar;13(2):134–148. doi: 10.1111/j.1600-0765.1978.tb00162.x. [DOI] [PubMed] [Google Scholar]
- Moretta L., Ferrarini M., Mingari M. C., Moretta A., Webb S. R. Subpopulations of human T cells identified by receptors for immunoglobulins and mitogen responsiveness. J Immunol. 1976 Dec;117(6):2171–2174. [PubMed] [Google Scholar]
- Moretta L., Webb S. R., Grossi C. E., Lydyard P. M., Cooper M. D. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med. 1977 Jul 1;146(1):184–200. doi: 10.1084/jem.146.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orstavik D., Brandtzaeg P. Serum antibodies to plaque bacteria in subjects with dental caries and gingivitis. Scand J Dent Res. 1977 Jan-Feb;85(2):106–113. doi: 10.1111/j.1600-0722.1977.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Page R. C., Clagett J. A., Engel L. D., Wilde G., Sims T. Effects of prostaglandin on the antigen- and mitogen-driven responses of peripheral blood lymphocytes from patients with adult and juvenile periodontitis. Clin Immunol Immunopathol. 1978 Sep;11(1):77–87. doi: 10.1016/0090-1229(78)90205-2. [DOI] [PubMed] [Google Scholar]
- Patters M. R., Genco R. J., Reed M. J., Mashimo P. A. Blastogenic response of human lymphocytes to oral bacterial antigens: comparison of individuals with periodontal disease to normal and edentulous subjects. Infect Immun. 1976 Nov;14(5):1213–1220. doi: 10.1128/iai.14.5.1213-1220.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Moretta L., Roper M., Breard J. M., Mingari M. C., Cooper M. D., Schlossman S. F. Human T lymphocyte subpopulations defined by Fc receptors and monoclonal antibodies. A comparison. J Exp Med. 1980 Apr 1;151(4):969–974. doi: 10.1084/jem.151.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trompeter R. S., Layward L., Hayward A. R. Primary and secondary abnormalities of T cell subpopulations. Clin Exp Immunol. 1978 Dec;34(3):388–392. [PMC free article] [PubMed] [Google Scholar]
- Victorino R. M., Hodgson H. J. Alteration in T lymphocyte subpopulations in inflammatory bowel disease. Clin Exp Immunol. 1980 Jul;41(1):156–165. [PMC free article] [PubMed] [Google Scholar]
- Weiner M. S., Bianco C., Nussenzweig V. Enhanced binding of neuraminidase-treated sheep erythrocytes to human T lymphocytes. Blood. 1973 Dec;42(6):939–946. [PubMed] [Google Scholar]


