Abstract
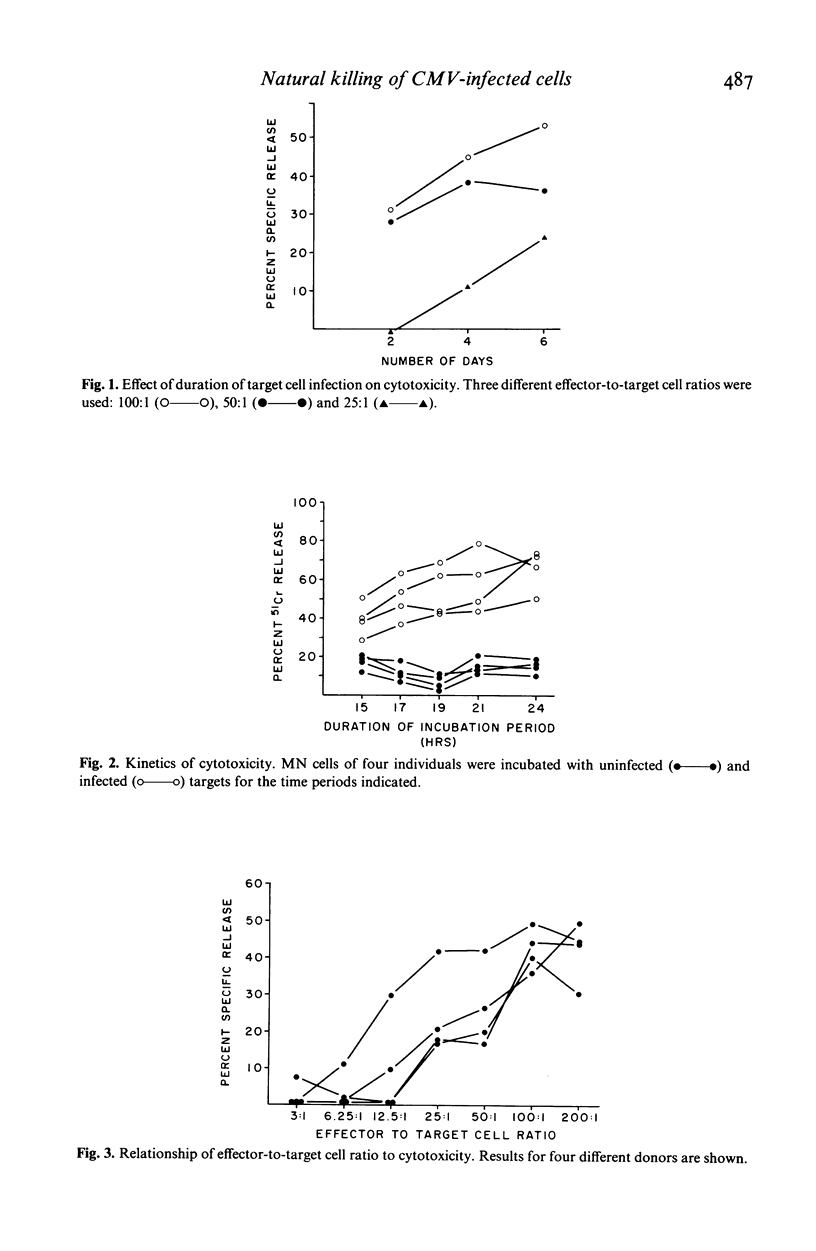
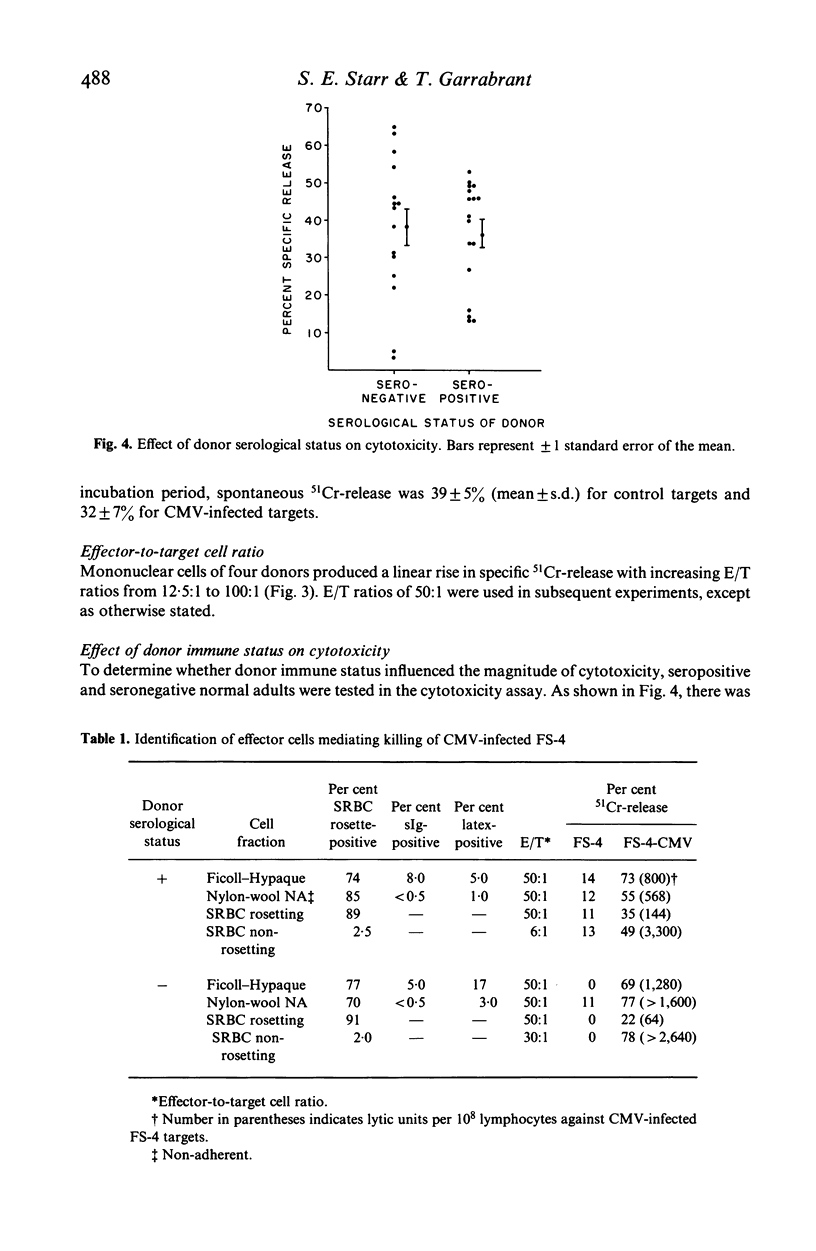
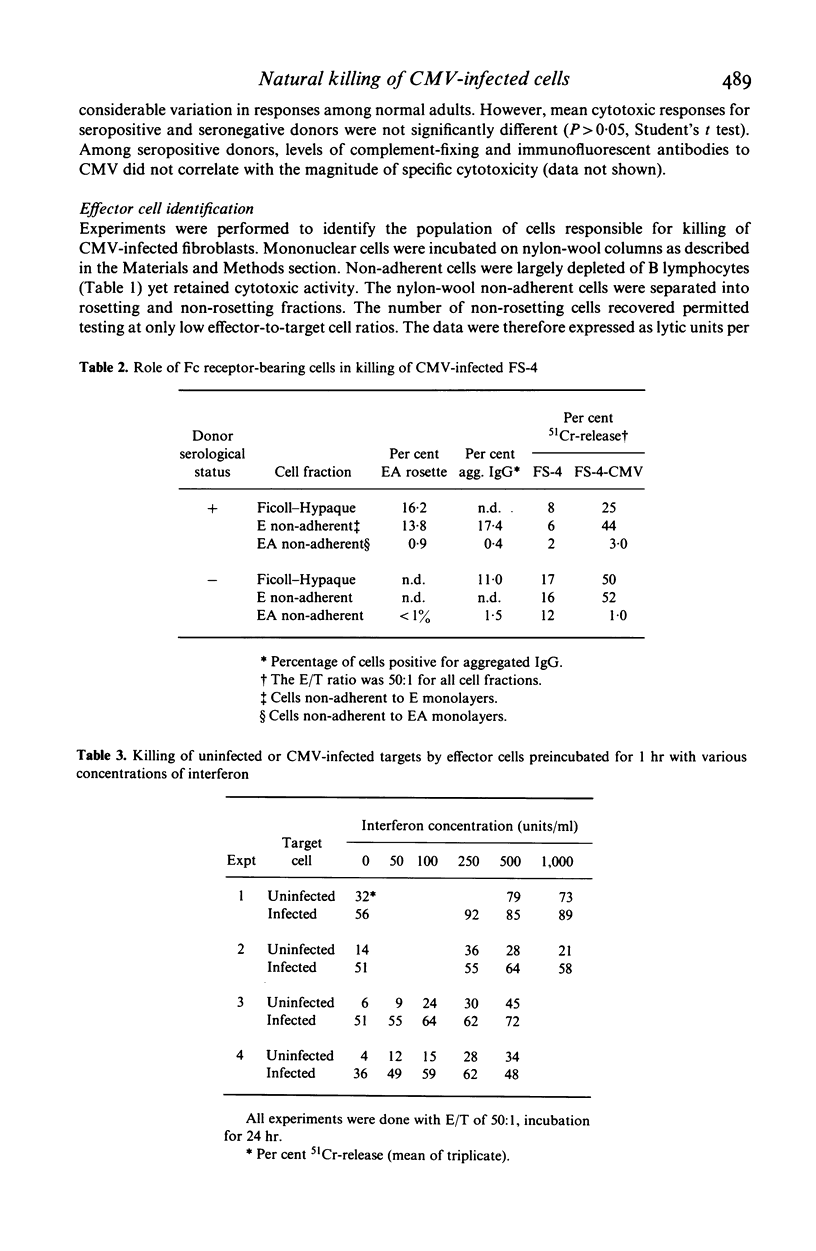
The ability of human peripheral blood mononuclear (MN) cells to lyse uninfected and cytomegalovirus (CMV) infected human fibroblasts was determined in a 51Cr-release assay. Maximal release was obtained with 6-day infected fibroblasts incubated with MN cells for 24 hr. A linear relationship existed between E/T ratios of 12.5:1 to 100:1 and lysis of CMV-infected targets. Donor immune status had no effect on the magnitude of killing of infected or uninfected targets. Killing was mediated by non-B, predominantly non-T, Fc receptor-bearing cells. Preincubation of effector cells with interferon enhanced killing of both CMV-infected and uninfected fibroblasts, but infected targets were more effectively killed. These results indicated a possible role for natural killer cells in recovery from CMV infection.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson T., Stejskal V., Harfast B. An in vitro method for study of human lymphocyte cytotoxicity against mumps-virus-infected target cells. J Immunol. 1975 Jan;114(1 Pt 1):237–243. [PubMed] [Google Scholar]
- Ault K. A., Weiner H. L. Natural killing of measles-infected cells by human lymphocytes. J Immunol. 1979 Jun;122(6):2611–2616. [PubMed] [Google Scholar]
- Cheeseman S. H., Rubin R. H., Stewart J. A., Tolkoff-Rubin N. E., Cosimi A. B., Cantell K., Gilbert J., Winkle S., Herrin J. T., Black P. H. Controlled clinical trial of prophylactic human-leukocyte interferon in renal transplantation. Effects on cytomegalovirus and herpes simplex virus infections. N Engl J Med. 1979 Jun 14;300(24):1345–1349. doi: 10.1056/NEJM197906143002401. [DOI] [PubMed] [Google Scholar]
- Ching C., Lopez C. Natural killing of herpes simplex virus type 1-infected target cells: normal human responses and influence of antiviral antibody. Infect Immun. 1979 Oct;26(1):49–56. doi: 10.1128/iai.26.1.49-56.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Keller R., Lee G., Finkel D. Lysis of cytomegalovirus-infected human fibroblasts and transformed human cells by peripheral blood lymphoid cells from normal human donors. Proc Soc Exp Biol Med. 1977 Feb;154(2):259–263. doi: 10.3181/00379727-154-39650. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Brown G. Purification of human T and B lymphocytes. J Immunol. 1974 Jan;112(1):420–423. [PubMed] [Google Scholar]
- Herberman R. B., Djeu J., Kay H. D., Ortaldo J. R., Riccardi C., Bonnard G. D., Holden H. T., Fagnani R., Santoni A., Puccetti P. Natural killer cells: characteristics and regulation of activity. Immunol Rev. 1979;44:43–70. doi: 10.1111/j.1600-065x.1979.tb00267.x. [DOI] [PubMed] [Google Scholar]
- Huddlestone J. R., Merigan T. C., Jr, Oldstone M. B. Induction and kinetics of natural killer cells in humans following interferon therapy. Nature. 1979 Nov 22;282(5737):417–419. doi: 10.1038/282417a0. [DOI] [PubMed] [Google Scholar]
- Kedar E., Ortiz de Landazuri M., Bonavida B. Cellular immunoadsorbents: a simplified technique for separation of lymphoid cell populations. J Immunol. 1974 Mar;112(3):1231–1243. [PubMed] [Google Scholar]
- Kohl S., Starr S. E., oleske J. M., Shore S. L., Ashman R. B., Nahmias A. J. Human monocyte-macrophage-mediated antibody-dependent cytotoxicity to herpes simplex virus-infected cells. J Immunol. 1977 Mar;118(3):729–735. [PubMed] [Google Scholar]
- Meyers J. D., McGuffin R. W., Neiman P. E., Singer J. W., Thomas E. D. Toxicity and efficacy of human leukocyte interferon for treatment of cytomegalovirus pneumonia after marrow transplantation. J Infect Dis. 1980 May;141(5):555–562. doi: 10.1093/infdis/141.5.555. [DOI] [PubMed] [Google Scholar]
- Santoli D., Koprowski H. Mechanisms of activation of human natural killer cells against tumor and virus-infected cells. Immunol Rev. 1979;44:125–163. doi: 10.1111/j.1600-065x.1979.tb00269.x. [DOI] [PubMed] [Google Scholar]
- Santoli D., Trinchieri G., Koprowski H. Cell-mediated cytotoxicity against virus-infected target cells in humans. II. Interferon induction and activation of natural killer cells. J Immunol. 1978 Aug;121(2):532–538. [PubMed] [Google Scholar]
- Sethi K. K., Stroehmann I., Brandis H. Human T-cell cultures from virus-sensitized donors can mediate virus-specific and HLA-restricted cell lysis. Nature. 1980 Aug 14;286(5774):718–720. doi: 10.1038/286718a0. [DOI] [PubMed] [Google Scholar]
- Shore S. L., Melewicz F. M., Gordon D. S. The mononuclear cell in human blood which mediates antibody-dependent cellular cytotoxicity to virus-infected target cells. I. Identification of the population of effector cells. J Immunol. 1977 Feb;118(2):558–566. [PubMed] [Google Scholar]
- Starr S. E. Cytomegalovirus. Pediatr Clin North Am. 1979 May;26(2):283–293. doi: 10.1016/s0031-3955(16)33705-1. [DOI] [PubMed] [Google Scholar]
- Starr S. E., Dalton B., Garrabrant T., Paucker K., Plotkin S. A. Lymphocyte blastogenesis and interferon production in adult human leukocyte cultures stimulated with cytomegalovirus antigens. Infect Immun. 1980 Oct;30(1):17–22. doi: 10.1128/iai.30.1.17-22.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thong Y. H., Hensen S. A., Vincent M. M., Fuccillo D. A., Stiles W. A., Bellanti J. A. Use of cryopreserved virus-infected target cells in a lymphocytotoxicity 51Cr release microassay for cell-mediated immunity to cytomegalovirus. Infect Immun. 1976 Feb;13(2):643–645. doi: 10.1128/iai.13.2.643-645.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G., Santoli D. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Enhancement of human natural killer cell activity by interferon and antagonistic inhibition of susceptibility of target cells to lysis. J Exp Med. 1978 May 1;147(5):1314–1333. doi: 10.1084/jem.147.5.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner M. S., Bianco C., Nussenzweig V. Enhanced binding of neuraminidase-treated sheep erythrocytes to human T lymphocytes. Blood. 1973 Dec;42(6):939–946. [PubMed] [Google Scholar]


