Abstract
The Plasmodium falciparum erythrocyte-binding antigen 175 (EBA-175) is a ligand for merozoite invasion into human erythrocytes that binds to glycophorin A in a sialic acid-dependent manner. P. falciparum strain W2mef depends on sialic acid for invasion of erythrocytes, whereas 3D7 is sialic acid-independent. We generated parasites that lack expression or express truncated forms of EBA-175 in W2mef and 3D7. Lack of EBA-175 expression in W2mef parasites was associated with a switch to sialic acid-independent invasion. 3D7 parasites lacking expression of EBA-175 showed no alteration in their ability to utilize sialic acid-independent pathways. Strikingly, both W2mef and 3D7 parasites lacking EBA-175 expression invaded chymotrypsin-treated erythrocytes inefficiently compared with the parental lines. This loss of function suggests that the EBA-175/glycophorin A ligand–receptor interaction is the major chymotrypsin-resistant invasion pathway. Parasite lines with truncated EBA-175 had invasion phenotypes equivalent to parasites lacking expression of EBA-175. The EBA-175 ligand is functional in erythrocyte invasion by merozoites that utilize either sialic acid-dependent or -independent invasion pathways. This finding suggests a model where a minimal affinity supplied by multiple ligand–receptor interactions is required for successful invasion and has implications for EBA-175 as a malaria vaccine candidate.
Plasmodium falciparum causes the most severe form of human malaria, with over two million deaths per year. The clinical symptoms of malaria result from exponential expansion of the parasite population during the asexual erythrocytic phase of the P. falciparum life cycle. The rapid nature of erythrocyte invasion by merozoites indicates that it is a tightly controlled process and involves specific receptor–ligand interactions between host and parasite molecules.
P. falciparum invades erythrocytes by using multiple receptor–ligand interactions defined as invasion pathways (1, 2). Receptors have been identified by using mutant erythrocytes and enzyme treatments and include glycophorin A, glycophorin B, and receptor “X” (1, 3, 4). The first P. falciparum ligand identified that binds to erythrocytes with high affinity was erythrocyte-binding antigen 175 (EBA-175, ref. 1). This protein has similarity to Plasmodium vivax Duffy-binding protein (PvDBP-1), the ligand that binds to the Duffy receptor during invasion of this parasite (5). EBA-175 and PvDBP-1 are members of a large family of proteins that share structural motifs in plasmodia that have been termed the Duffy-binding-like-erythrocyte-binding protein family (6). A family of genes homologous to EBA-175 has been identified from the P. falciparum genome (7) and a member of this family, EBA-140 (BAEBL), has been shown to bind erythrocytes (8–10). EBA-140 binds to glycophorin C and functions in an invasion pathway through this receptor (11), and it has been suggested that polymorphisms alter affinity and perhaps receptor specificity (11, 12).
EBA-175 binds to glycophorin A on the surface of erythrocytes, and this interaction depends on sialic acid on the receptor (13). Treatment of erythrocytes with neuraminidase ablates EBA-175 binding by removal of sialic acid (1). The binding region of EBA-175 to glycophorin A involves the cysteine-rich region II consisting of two halves called F1 and F2 domains. Abs to the F2 domain of EBA-175 can partially inhibit invasion of P. falciparum merozoites into human erythrocytes (14, 15).
It has been shown that some strains of P. falciparum cannot invade neuraminidase-treated erythrocytes and depend on sialic acid receptors for invasion (1, 16). However, other P. falciparum strains are able to invade via alternative pathways into neuraminidase-treated erythrocytes and are sialic acid-independent (17). The sialic acid-dependent strain W2mef can be selected for invasion in a sialic acid-independent manner for invasion by growth on neuraminidase-treated erythrocytes, demonstrating these parasites possess the potential to invade via different pathways (2). Construction of a W2mef parasite line that expresses truncated EBA-175 resulted in a switch to sialic acid-independent invasion (18).
Here we have used two P. falciparum lines that invade via sialic acid-dependent or -independent invasion pathways to generate parasites that either do or do not express truncated versions of EBA-175. We find that EBA-175 is functional in both sialic acid-dependent and -independent parasite lines, and that glycophorin A is the dominant chymotrypsin-resistant receptor for invasion. These results suggest a model for sialic acid dependence and independence of invasion.
Methods
Plasmids and Parasites.
To disrupt EBA-175, 5′ and 3′ sequences were cloned into pHTk to derive pHTkΔ175 (19). Flank 1 was amplified from genomic DNA by using primers 5′-GGACCccgcggTCAATGTGCATACAATGTATTGAAATGA and 5′-GGACCagatctCTACGATCAGGAATACATACATAGTTG (lowercase indicating relevant restriction enzyme cleavage sites) and cloned into pHTk by using SacII/BglII to give pHTk-EBA-F1. Flank 2 was amplified by using the primers 5′-GGACCcctaggATAAGAAATAATGAACAAACTTCGCAAGA and 5′-GGACCcccgggATGTAGATTATTCATGGTATGGAATCCATGT and cloned into the XmaI/AvrII site of pHCD-rh3 to yield pHCD-rh3-5′-175-3′. The EBA-175 flank 2 was then subcloned from this plasmid into the AatII/EcoRI fragment of the vector pHTk-EBAF1 to yield pHTkΔ175.
3D7 was obtained from D. Walliker (Edinburgh University, Edinburgh). W2mef is a cloned line from the Indochina III/CDC. Transfection with 80 μg of plasmid DNA (Qiagen, Valencia, CA) and selection for transfectants with double-recombination crossover was performed as described (19). To obtain an EBA-175 gene truncation by single-crossover recombination, pHTkΔ175 was used.
SDS/PAGE and Immunoblot Analysis.
Erythrocytes containing synchronized parasites (5–10% parasitemia) were treated with neuraminidase (66.7 milliunits/ml)/trypsin (1 mg/ml) to prevent reinvasion, and the culture supernatant was collected. The supernatants were centrifuged at 1,500 rpm (Beckman GS-6KR). Proteins were separated on SDS/6% PAGE followed by blotting to nitrocellulose (0.45 μm, Schleicher & Schuell). After appropriate antibody incubation, blots were processed by enhanced chemiluminescence (ECL, Amersham Biosciences).
Overlay Assays.
EBA-175 and EBA-140 binding to erythrocyte membrane proteins was as described (11, 20). Erythrocyte membranes were prepared by hypotonic lysis, and the proteins (30 μg per lane ≈5 × 107 cells) were separated by SDS/10% PAGE and transferred to nitrocellulose (0.45 μm, Schleicher & Schuell). Abs used for antigen detection were anti-EBA-175 (18) and anti-EBA-140 (9).
Erythrocyte Invasion Assay.
The invasion assay was carried out as described (18). Uninfected erythrocytes were treated with enzymes (9). Erythrocytes infected with synchronized rings were treated with trypsin (1 mg/ml) and neuraminidase (66.7 milliunits/ml) to prevent reinvasion. Experiments were done with the addition of new erythrocytes treated with trypsin (66.7 μg/ml), neuraminidase (66.7 milliunits/ml), or chymotrypsin (1 mg/ml). Experiments were performed in triplicate. Enzyme-treated or untreated erythrocytes at 4% hematocrit were inoculated with infected erythrocytes to give a parasitemia of 0.5% in 100 μl per well. Parasites were incubated 48 h, after which [3H]hypoxanthine (Amersham Biosciences) was added to 1 μCi per well (1 Ci = 37 GBq). After 16 h the cells were harvested (Packard). Incorporated [3H]hypoxanthine was determined in a scintillation counter. Percentage invasion was calculated by comparing invasion of the same parasite line into treated vs. untreated erythrocytes. Invasion rates were between 3% and 7% for untreated erythrocytes.
Results
EBA-175 Can Be Disrupted in P. falciparum by Using Sialic Acid-Dependent or -Independent Invasion Pathways.
To test the role of EBA-175 in parasites using sialic acid-dependent (W2mef) and -independent (3D7) invasion pathways, we constructed parasites that express or do not express truncated protein. The pHTkΔ175 vector contains the thymidine kinase gene for negative selection and the human DHFR gene conferring resistance to WR99210 (Fig. 1B; ref. 19). The hDHFR gene was flanked by two sequences from EBA-175 for homologous recombination (Fig. 1A; ref. 3). Both 3D7 and W2mef were transfected with pHTkΔ175 and selected on WR99210. These parasites were then selected with WR99210 and ganciclovir to obtain parasites that integrated the hDHFR cassette by double-crossover homologous recombination (19). The 3D7 and W2mef double-crossover recombinant parasites were cloned to derive 3D7Δ175/1, 3D7Δ175/2, W2mefΔ175/1, and W2mefΔ175/2.
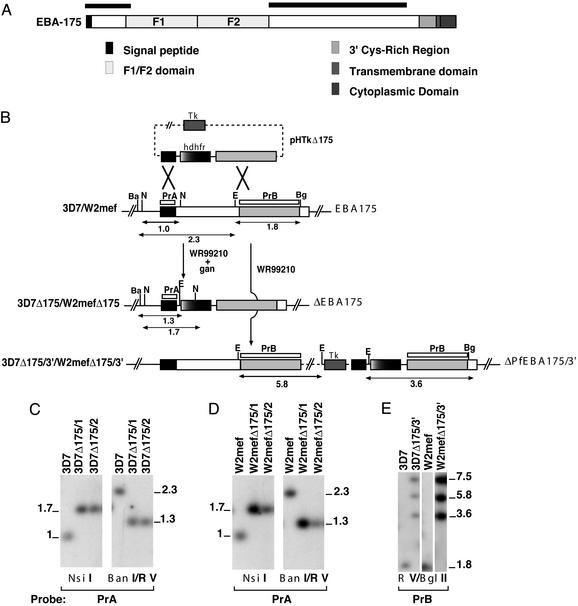
Figure 1.
Disruption of EBA-175 in two lines of P. falciparum that invade by either sialic acid-dependent or -independent pathways. (A) Structure of EBA-175. Shown are signal sequence, F1 and F2 domains that bind to glycophorin A, 3′ cysteine-rich region, transmembrane domain, and cytoplasmic domain. The black boxes above the protein refer to regions where crossover occurred in the gene sequence. This crossover resulted in deletion of the F1/F2 region from the genome. (B) Disruption of the EBA-175 gene in 3D7 and W2mef. The pHTkΔ175 plasmid contains the thymidine kinase gene (Tk), hDHFR, and 5′ and 3′ EBA-175 regions. EBA-175 is shown with homologous target sequences in black (5′ flank) and gray (3′ flank). The double-crossover integration events are shown for both 3D7Δ175 and W2mefΔ175 where the F1/F2 region has been deleted. The single-crossover recombination events for 3D7Δ175/3′ and W2mefΔ175/3′ are shown where one copy of the full transfection plasmid has integrated. Sizes of DNA fragments are shown in kb. Restriction enzyme sites are N, NsiI; Ba, BanI; E, EcoRV; and Bg, BglII. PrA and PrB are hybridization probes used in C–E. (C) Southern blot of genomic DNA from parasites shown digested with NsiI or BanI/EcoRV and probed with PrA. (D) Southern blot of genomic DNA from the parasites shown digested with NsiI or BanI/EcoRV and probed with PrA. (E) Southern blot of genomic DNA from the parasites shown digested with EcoRV or BglII and probed with PrB. Sizes of fragments are in kbp.
To confirm that pHTKΔ175 had integrated into EBA-175, genomic DNA from parental and recombinant parasites was digested with NsiI and BanI/EcoRV and probed with the 5′ region of EBA-175 (PrA; Fig. 1 C and D). This hybridization revealed 1.0- and 2.3-kb fragments for the NsiI and BanI/EcoRV digests, respectively, in the parental lines corresponding to endogenous EBA-175. 3D7Δ175/1, 3D7Δ175/2, and W2mefΔ175/1 and 2 had hybridizing bands of the expected size, confirming that EBA-175 had been disrupted in the four cloned lines by double-crossover recombination. This integration event led to the deletion of the F1/F2 region responsible for glycophorin A binding.
EBA-175 has been disrupted in W2mef, resulting in expression of a truncated protein lacking the 3′ cysteine-rich region, transmembrane domain, and the cytoplasmic domain (18). This recombinant W2mef parasite showed a switch in invasion to sialic acid independence. We sought to determine the effect of a similar mutation in 3D7, a parasite line that utilizes sialic acid-independent pathways. W2mef and 3D7 parasites transfected with pHTkΔ175 were cycled on WR99210 to select for recombinant parasites that had integrated the plasmid by a single-recombination crossover event at the 3′ end (Fig. 1B). Southern hybridization using EcoRV/BglII-digested genomic DNA from parental and transfected parasites confirmed that pHTkΔ175 had integrated into EBA-175 to give 3D7Δ175/3′ and W2mefΔ175/3′. Hybridization with a probe to the 3′ flank (PrB) revealed a 1.8-kb fragment in both parental lines corresponding to endogenous EBA-175. However, in 3D7Δ175/3′ and W2mefΔ175/3′, three hybridizing bands of 7.5, 5.8, and 3.6 kb were observed, which was consistent with integration of the plasmid into EBA-175 by a homologous single-crossover recombination into the 3′ flank.
Expression of EBA-175 in the Recombinant Lines.
To determine whether integration of pHTkΔ175 into EBA-175 of 3D7Δ175 and W2mefΔ175 had blocked expression of the protein, we used supernatants probed with Abs specific to EBA-175 (Fig. 2A; ref. 18). EBA-175 protein was detectable in both W2mef and 3D7 as expected. In contrast, the 3D7Δ175/1 and 2 and W2mefΔ175/1 and 2 supernatants showed no expression of EBA-175 (Fig. 2A), and similar experiments with pellets of mature schizonts showed no expression of the protein (data not shown). Therefore, disruption of EBA-175 in 3D7Δ175/1 and 2 and W2mefΔ175/1 and 2 resulted in lack of expression of the EBA-175 protein.
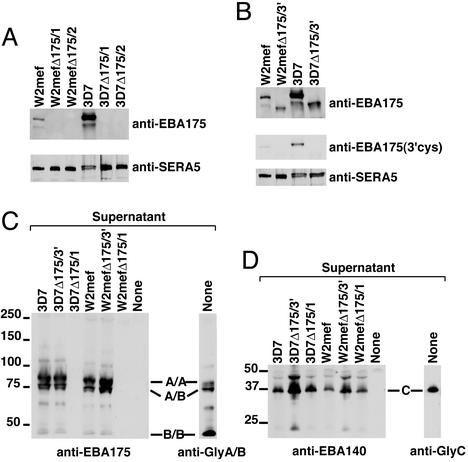
Figure 2.
Expression and binding of EBA-175 in P. falciparum lines to erythrocyte proteins. (A) Western blot of supernatants from W2mef, W2mefΔ175/1 and 2, 3D7, 3D7Δ175/1, and 3D7Δ175/1 and 2 probed with anti-EBA-175 (Upper) Abs (17). (Lower) A control of the same supernatants probed with anti-SERA5 Abs. (B) Western blot of supernatants from W2mef, W2mefΔ175/3′, 3D7, and 3D7Δ175/3′ probed with anti-EBA-175 Abs (Top) or 3′-Cys Abs (Middle). A control of the same supernatants is shown probed with anti-SERA5 (Bottom). (C) Membrane proteins from erythrocyte ghosts separated by SDS/PAGE and blotted to nitrocellulose. The filters were incubated with supernatants from 3D7, 3D7Δ175/3′, 3D7Δ175/1, W2mef, W2mefΔ175/3′, W2mefΔ175/1, or no supernatant (None). After washing, the incubated filter was probed with anti-EBA-175 Abs to detect bound ligand. The track on the right shows the erythrocyte membrane proteins probed with anti-glycophorin A/B Abs to show the glycophorin homodimer (A/A), glycophorin A and B heterodimer (A/B), and the glycophorin B homodimer (B/B). (D) Erythrocyte ghost proteins prepared as in C and incubated with the same supernatants but probed with anti-EBA-140 (BAEBL) Abs. The track labeled “None” was not incubated with supernatant but probed with anti-EBA-140 Abs to show nonspecific crossreactivity of the Ab with erythrocyte membrane proteins. The track on the right is the erythrocyte ghost proteins probed with anti-glycophorin C.
Immunoblot analysis of supernatants from W2mefΔ175/3′ and 3D7Δ175/3′ showed expression of EBA-175; however, it was smaller compared with that observed in W2mef and 3D7 parents. Probing of identical immunoblots with Abs specific for the 3′ cysteine-rich region (18) recognized EBA-175 in supernatants from wild-type parasites but no band was observed in either W2mefΔ175/3′ or 3D7Δ175/3′. This immunoblot confirmed that integration of the pHTkΔ175 plasmid had resulted in disruption of the gene and expression of a truncated protein lacking the 3′ cysteine-rich region and transmembrane and cytoplasmic domains.
Full-Length and Truncated EBA-175 Proteins Bind to Glycophorin A.
To confirm that EBA-175 from parental parasites and the truncated protein in W2mefΔ175/3′ and 3D7Δ175/3′ bound to glycophorin A, we performed overlay experiments on membranes of erythrocytes (Fig. 2C). As expected, EBA-175 from 3D7 and W2mef bound to the homodimer of glycophorin A and the heterodimer of glycophorins A and B. Similarly, the truncated EBA-175 protein from W2mefΔ175/3′ and 3D7Δ175/3′ bound to the glycophorin A homodimer and the glycophorin A and B heterodimer. This binding shows that even in the absence of a large portion of the C terminus of the protein, the F1/F2 domains of W2mefΔ175/3′ and 3D7Δ175/3 possess erythrocyte-binding activity. In contrast to the truncated proteins, supernatants from 3D7Δ175/1 and W2mefΔ175/1 showed no binding in overlay experiments, consistent with lack of expression of EBA-175 in these parasites. As a control, EBA-140 (BAEBL) present in all of the supernatants was tested and bound efficiently to glycophorin C (Fig. 2D; ref. 11).
EBA-175 Functions in Parasite Lines That Invade by Either Sialic Acid-Independent or -Dependent Merozoite Invasion Pathways.
It has been shown that truncation of EBA-175 in W2mef results in an apparent switch in merozoite invasion from utilization of sialic acid-dependent invasion pathways to sialic acid-independent pathways (18). We have confirmed this finding by comparison of the efficiency of merozoite invasion of the parental W2mef with W2mefΔ175/3′, which expresses the truncated form of EBA-175. Comparison of W2mef and W2mefΔ175 invasion into untreated erythrocytes was approximately the same as that observed when 3D7 was compared with 3D7Δ175 (data not shown). This equivalent efficiency of merozoite invasion for wild-type and mutant parasites suggested that other invasion pathways had compensated for loss of EBA-175 function or that these ligands were not critical. W2mef showed very low levels of invasion for neuraminidase-treated erythrocytes (6.6 ± 1.4%), whereas W2mefΔ175/3′ invaded the same treated erythrocytes at high efficiency (62 ± 12%), confirming an apparent switch to sialic acid-independent merozoite invasion (ref. 18; Fig. 3). We then compared the effect of loss of EBA-175 expression in 3D7, a parasite line that invades primarily in a sialic acid-independent manner. The 3D7 and the 3D7Δ175/3′ merozoites showed similar levels of invasion into neuraminidase-treated erythrocytes (76.5 ± 8.8% compared with 86 ± 9%), showing that truncation of EBA-175 in 3D7 had no effect on sialic acid-independent invasion (Fig. 3).
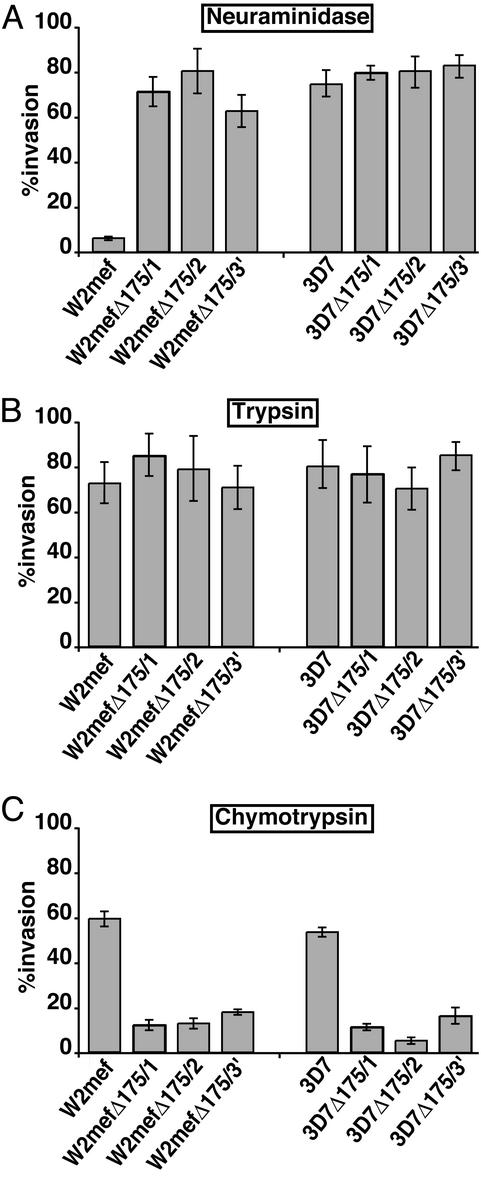
Figure 3.
Comparison of merozoite invasion of mutant P. falciparum into enzyme-treated erythrocytes. Shown is merozoite invasion of W2mef, W2mefΔ175/1, W2mefΔ175/2, W2mefΔ175/3′, 3D7, 3D7Δ175/1, 3D7Δ175/2, and 3D7Δ175/3′ into neuraminidase- (A), trypsin- (B), or chymotrypsin (C)-treated erythrocytes. Invasion shown is relative to invasion of parental controls into untreated erythrocytes with 95% confidence levels indicated. Values represent 4–10 independent experiments, each done in triplicate. Assays were plated at 0.5% parasitemia, and the invasion rate for untreated erythrocytes was between 3% and 7% when sampled.
The ability of recombinant parasites lacking EBA-175 to invade neuraminidase-treated erythrocytes was measured. W2mefΔ175/1 and 2 invaded erythrocytes lacking sialic acid (70.6 ± 10.9% and 75.5 ± 15.3%, respectively), in contrast to low levels of invasion observed for W2mef. Therefore, both EBA-175-null parasites W2mefΔ175/1 and W2mefΔ175/2 have the same apparent switch to a sialic acid-independent pathway as the EBA-175-truncated parasite W2mefΔ175/3′. This switch of invasion pathways suggests that EBA-175 is a dominant parasite ligand for sialic acid-dependent invasion in W2mef. In contrast, 3D7Δ175/1 and 3D7Δ175/2 (80.4 ± 5.6% and 81.3 ± 10.5%, respectively), like the EBA-175-truncated parasite 3D7Δ175/3′, show no alteration in invasion of neuraminidase-treated erythrocytes compared with 3D7 (76.5 ± 8.8%). Therefore, loss of function of EBA-175 in a sialic acid-independent parasite line has no apparent effect on invasion into erythrocytes.
Glycophorin A is trypsin-sensitive, and treatment of erythrocytes ablates the ability of P. falciparum to invade via this receptor (17). In contrast, the same receptor resists treatment with chymotrypsin, whereas other receptors such as glycophorin B are sensitive (9). Treatment of erythrocytes with either trypsin or chymotrypsin limits the receptor repertoire for invasion. We tested the ability of the different parasite lines to invade protease-treated erythrocytes. Comparison of the parental 3D7 and W2mef with the different recombinant parasite lines showed no differences in invasion into trypsin-treated erythrocytes, consistent with the sensitivity of glycophorin A to this protease. The presence or absence of EBA-175 makes no difference if the receptor has already been removed. In contrast, a large difference in efficiency of invasion into chymotrypsin-treated erythrocytes was observed between the parental lines and the recombinant lines. W2mef (59.5 ± 5.1%) and 3D7 (53.8 ± 7.2%) were able to invade chymotrypsin-treated erythrocytes efficiently, whereas 3D7Δ175/1, 3D7Δ175/2, W2mefΔ175/1, and W2mefΔ175/2, which lack expression of EBA-175, showed low levels of invasion. Similarly, W2mefΔ175/3′ and 3D7Δ175/3′ also showed low levels of merozoite invasion into chymotrypsin-treated erythrocytes. Taken together these results show (i) that lack of expression or truncation of EBA-175 causes a loss of function of this protein in parasite lines that invade by using either sialic acid-independent or -dependent pathways, and (ii) that glycophorin A is the dominant chymotrypsin-resistant receptor on the erythrocyte for merozoite invasion. Therefore EBA-175 is functional in both W2mef and 3D7 parasite lines, which use sialic acid-dependent and -independent pathways, respectively.
Discussion
The invasion of P. falciparum merozoites into human erythrocytes requires specific ligand–receptor interactions. EBA-175 has been implicated as a ligand that binds to sialic acid residues of glycophorin A (1, 3, 4). This ligand–receptor interaction has defined an invasion pathway utilizing EBA-175 and glycophorin A; however, it is clear that there are other pathways mediated by additional ligand–receptor interactions (17), some of which are sialic acid-independent. We have disrupted the EBA-175 gene in P. falciparum to investigate the role of EBA-175 in parasites that invade by either sialic acid-dependent or -independent pathways. We show that EBA-175 is functional in parasites that invade by using either sialic acid-dependent or -independent pathways. Invasion into erythrocytes with their repertoire of receptors limited by chymotrypsin treatment is greatly reduced when EBA-175 is removed, showing that glycophorin A is the dominant chymotrypsin-resistant receptor for merozoite invasion. Therefore, the EBA-175-mediated invasion pathway is more significant than the chymotrypsin-resistant EBA-140/glycophorin C pathway, which we have demonstrated to be functional in these parasite lines (11). Our results are also consistent with data showing that truncation of EBA-175 in W2mef was associated with an apparent switch toward a sialic acid-independent pathway of invasion (18). Removal of the 3′ cysteine-rich region, cytoplasmic tail, and transmembrane region of EBA-175 is equivalent to lack of expression of the protein, and these regions are essential for EBA-175 function in both W2mef and 3D7 (18).
The apparent switch of W2mef to sialic acid-independent invasion as a result of the absence of EBA-175 or truncation in the W2mef recombinant parasites is consistent with EBA-175 being a dominant ligand for sialic acid-dependent invasion in this parasite line. This dominance of EBA-175 is in contrast to 3D7, which can efficiently invade neuraminidase-treated erythrocytes and suggests that the major ligand–receptor interactions in 3D7 invasion are not sialic acid-dependent despite the presence of functional EBA-175. The sialic acid-independent ligand–receptor interactions in 3D7 invasion are dominant over EBA-175 function.
A model for receptor–ligand interactions has been developed that can explain the sialic acid-dependence and -independence of parasite lines such as W2mef and 3D7 for merozoite invasion into erythrocytes (Fig. 4). The W2mef line relies for merozoite invasion primarily on ligands binding to erythrocyte receptors in a sialic acid-dependent manner. Treatment of erythrocytes with neuraminidase removes these receptors on the erythrocyte surface, and the remaining interactions available are sialic acid-independent; however, there would be insufficient affinity for these ligand–receptor interactions to mediate efficient invasion (Fig. 4). Invasion of W2mef into chymotrypsin-treated erythrocytes is efficient because glycophorin A is chymotrypsin-resistant, allowing interaction of EBA-175. In contrast, 3D7 uses predominantly sialic acid-independent ligands but also expresses sialic acid-dependent ligands such as EBA-175 that function in invasion. Invasion of 3D7 into neuraminidase-treated erythrocytes is efficient because of the predominance of sialic acid-independent ligands. Similarly, invasion into chymotrypsin-treated erythrocytes is possible, because of the presence of functional EBA-175 ligand binding to the chymotrypsin-resistant receptor glycophorin A.
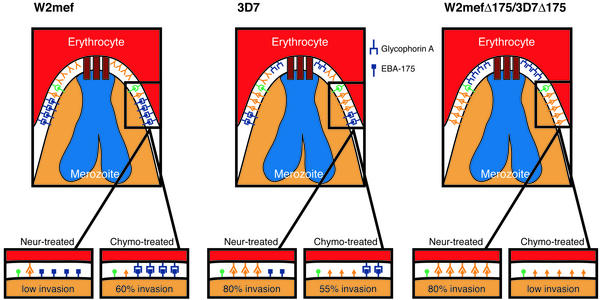
Figure 4.
A model for invasion via sialic acid-dependent and -independent pathways in P. falciparum. The erythrocyte and invading merozoite are shown for W2mef, 3D7 W2mefΔ175, and 3D7Δ175. The red blocks at the apical end of each merozoite represent interaction with the PfRh proteins with their specific ligands, and this ligand–receptor interaction is a prerequisite for tight junction formation, perhaps by inducing release of EBA-175 and other ligands from the micronemes (22). After apical interaction the tight junction is formed by recruitment of high-affinity ligands such as EBA-175 that bind to the chymotrypsin-resistant receptor glycophorin A. In W2mef, EBA-175 is the dominant ligand, treatment of erythrocytes with neuraminidase (Neur-treated) removes all sialic acid, and the only ligands that can interact are sialic acid-independent. For W2mef there are not enough sialic acid-independent ligands to reach the required threshold of affinity to mediate invasion of neuraminidase-treated erythrocytes. Because glycophorin A is chymotrypsin-resistant, W2mef is able to invade erythrocytes treated with this protease through the EBA-175/glycophorin A pathway. 3D7 invades by using neuraminidase-independent pathways; however, EBA-175 is functional, so the parasite can invade both chymotrypsin- and neuraminidase-treated erythrocytes efficiently. The W2mefΔ175 and 3D7Δ175 parasites lacking EBA-175 show the same invasion phenotypes. They invade neuraminidase-treated erythrocytes through the ligand that replaced EBA-175 but cannot, however, invade chymotrypsin-treated erythrocytes because of the absence of EBA-175.
Disruption of EBA-175 in W2mef has selected for parasites that invade via sialic acid-independent pathways so that these transfected parasites have the same invasion phenotype as ΔEBA-175 3D7 parasites. A similar switch in invasion has been observed for truncation of EBA-175 in W2mef (18) and also by direct selection of the same parasite line on neuraminidase-treated erythrocytes (2). A similar EBA-175 truncation has been constructed in a cloned line of W2mef parasites (Dd2) selected on neuraminidase-treated erythrocytes (Dd2/Nm), and the transfected lines derived from this transfection show the same ability to efficiently invade neuraminidase-treated erythrocytes as the Dd2/Nm parasite line (21). The Dd2/Nm parasites are already able to invade primarily by sialic acid-independent pathways and therefore it would be expected that loss of function of EBA-175 would have no effect on this phenotype. However, we would predict that the Dd2/Nm parasite line with a truncated EBA-175 would show a greatly decreased ability to invade chymotrypsin-treated erythrocytes because of loss of EBA-175 ligand function.
The apparent switch of W2mefΔ175 parasites to sialic acid-independent invasion because of loss of EBA-175 function can be explained by two possible mechanisms. First, loss of function of EBA-175 may select for parasites with a genetic switch activating the expression of another parasite ligand that binds to a receptor on the erythrocyte in a sialic acid-independent manner. This protein may be the dominant sialic acid-independent ligand expressed in 3D7. Second, it is possible that lack of expression of EBA-175 in W2mef allows a second ligand from an excess pool to move into the tight junction formed between the parasite and the erythrocyte. This area is a finite space and it is likely that only a portion of the available ligands are utilized for interaction with receptors on the erythrocyte. The sialic acid-independent ligand(s) that replaces EBA-175 remains to be identified.
Abs to the F2 domain of EBA-175, which mediates binding to glycophorin A, are able to partially inhibit merozoite invasion of P. falciparum into human erythrocytes (14, 15). The same Abs can interfere with invasion by parasites that use sialic acid- independent pathways, such as 3D7 (14). This antibody inhibition can be explained by the observation that EBA-175 is functional in 3D7. However, it is clear that EBA-175 function is not required for normal merozoite invasion in either W2mef or 3D7. It is likely that there would be an advantage for the parasite to have several different potential ligands to mediate invasion. First, multiple ligand–receptor interactions may be required to provide a sufficiently high affinity for invasion. Second, loss of pathways such as the EBA-175/glycophorin A would be disadvantageous in a natural infection if other pathways were not present to compensate for its loss. Both receptor polymorphism and immunity against individual ligands would lead to their inability to contribute to the invasion process. Finally, P. falciparum parasites lacking functional EBA-175 may arise under immune selection in humans from use of this protein as a vaccine. It will be important to take this into account if this protein is to go forward as a vaccine candidate, and these results strongly support the idea of using EBA-175 in combination with other proteins involved in non-sialic acid invasion pathways.
Acknowledgments
We thank Brendan Crabb and Susanne Miller for SERA5 antibodies, and the Red Cross Blood Service (Melbourne) for supplying red cells and serum. This work was supported by the National Health and Medical Research Council of Australia. M.T.D. is funded by a Wellcome Trust Advanced Training Fellowship (Tropical Medicine), A.G.M. by a Deutsche Forschungsgemeinschaft research fellowship, and A.F.C. by a Howard Hughes international scholarship.
Abbreviation
- EBA
erythrocyte-binding antigen
References
- 1.Camus D, Hadley T J. Science. 1985;230:553–556. doi: 10.1126/science.3901257. [DOI] [PubMed] [Google Scholar]
- 2.Dolan S A, Miller L H, Wellems T E. J Clin Invest. 1990;86:618–624. doi: 10.1172/JCI114753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sim B K L, Orlandi P A, Haynes J D, Klotz F W, Carter J M, Camus D, Zegans M E, Chulay J D. J Cell Biol. 1990;111:1877–1884. doi: 10.1083/jcb.111.5.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dolan S A, Proctor J L, Alling D W, Okubo Y, Wellems T E, Miller L H. Mol Biochem Parasitol. 1994;64:55–63. doi: 10.1016/0166-6851(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 5.Adams J H, Hudson D E, Torii M, Ward G E, Wellems T E, Aikawa M, Miller L H. Cell. 1990;63:141–153. doi: 10.1016/0092-8674(90)90295-p. [DOI] [PubMed] [Google Scholar]
- 6.Adams J H, Sim B K L, Dolan S A, Fang X, Kaslow D C, Miller L H. Proc Natl Acad Sci USA. 1992;89:7085–7089. doi: 10.1073/pnas.89.15.7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams J H, Blair P L, Kaneko O, Peterson D S. Trends Parasitol. 2001;17:297–299. doi: 10.1016/s1471-4922(01)01948-1. [DOI] [PubMed] [Google Scholar]
- 8.Mayer D C, Kaneko O, Hudson-Taylor D E, Reid M E, Miller L H. Proc Natl Acad Sci USA. 2001;98:5222–5227. doi: 10.1073/pnas.081075398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson J K, Triglia T, Reed M B, Cowman A F. Mol Microbiol. 2001;41:47–58. doi: 10.1046/j.1365-2958.2001.02484.x. [DOI] [PubMed] [Google Scholar]
- 10.Narum D L, Fuhrmann S R, Luu T, Sim B K. Mol Biochem Parasitol. 2002;119:159–168. doi: 10.1016/s0166-6851(01)00428-5. [DOI] [PubMed] [Google Scholar]
- 11.Maier A G, Duraisingh M T, Reeder J C, Patel S S, Kazura J W, Zimmerman P A, Cowman A F. Nat Med. 2003;9:87–92. doi: 10.1038/nm807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayer D C, Mu J B, Feng X, Su X Z, Miller L H. J Exp Med. 2002;196:1523–1528. doi: 10.1084/jem.20020750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sim B K L, Chitnis C E, Wasniowska K, Hadley T J, Miller L H. Science. 1994;264:1941–1944. doi: 10.1126/science.8009226. [DOI] [PubMed] [Google Scholar]
- 14.Narum D L, Haynes D, Fuhrmann S, Moch K, Liang H, Hoffman S L, Sim B K L. Infect Immun. 2000;68:1964–1966. doi: 10.1128/iai.68.4.1964-1966.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandey K C, Singh S, Pattnaik P, Pillai C R, Pillai U, Lynn A, Jain S K, Chitnis C E. Mol Biochem Parasitol. 2002;123:23–33. doi: 10.1016/s0166-6851(02)00122-6. [DOI] [PubMed] [Google Scholar]
- 16.Orlandi P A, Sim K L, Chulay J D, Haynes J D. Mol Biochem Parasitol. 1990;40:285–294. doi: 10.1016/0166-6851(90)90050-v. [DOI] [PubMed] [Google Scholar]
- 17.Hadley T J, Klotz F W, Pasvol G, Haynes J D, McGinniss M H. J Clin Invest. 1987;80:1190–1193. doi: 10.1172/JCI113178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reed M B, Caruana S R, Batchelor A H, Thompson J K, Crabb B S, Cowman A F. Proc Natl Acad Sci USA. 2000;97:7509–7514. doi: 10.1073/pnas.97.13.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duraisingh M T, Triglia T, Cowman A F. Int J Parasitol. 2002;32:81–89. doi: 10.1016/s0020-7519(01)00345-9. [DOI] [PubMed] [Google Scholar]
- 20.Nieper H, Muller H. J Gen Virol. 1996;77:1229–1237. doi: 10.1099/0022-1317-77-6-1229. [DOI] [PubMed] [Google Scholar]
- 21.Kaneko O, Fidock D A, Schwartz O M, Miller L H. Mol Biochem Parasitol. 2000;110:135–146. doi: 10.1016/s0166-6851(00)00263-2. [DOI] [PubMed] [Google Scholar]
- 22.Duraisingh M T, Triglia T, Stuart A R, Rayner J C, Barnwell J W, McFadden G I, Cowman A F. EMBO J. 2003;22:1047–1057. doi: 10.1093/emboj/cdg096. [DOI] [PMC free article] [PubMed] [Google Scholar]