Abstract
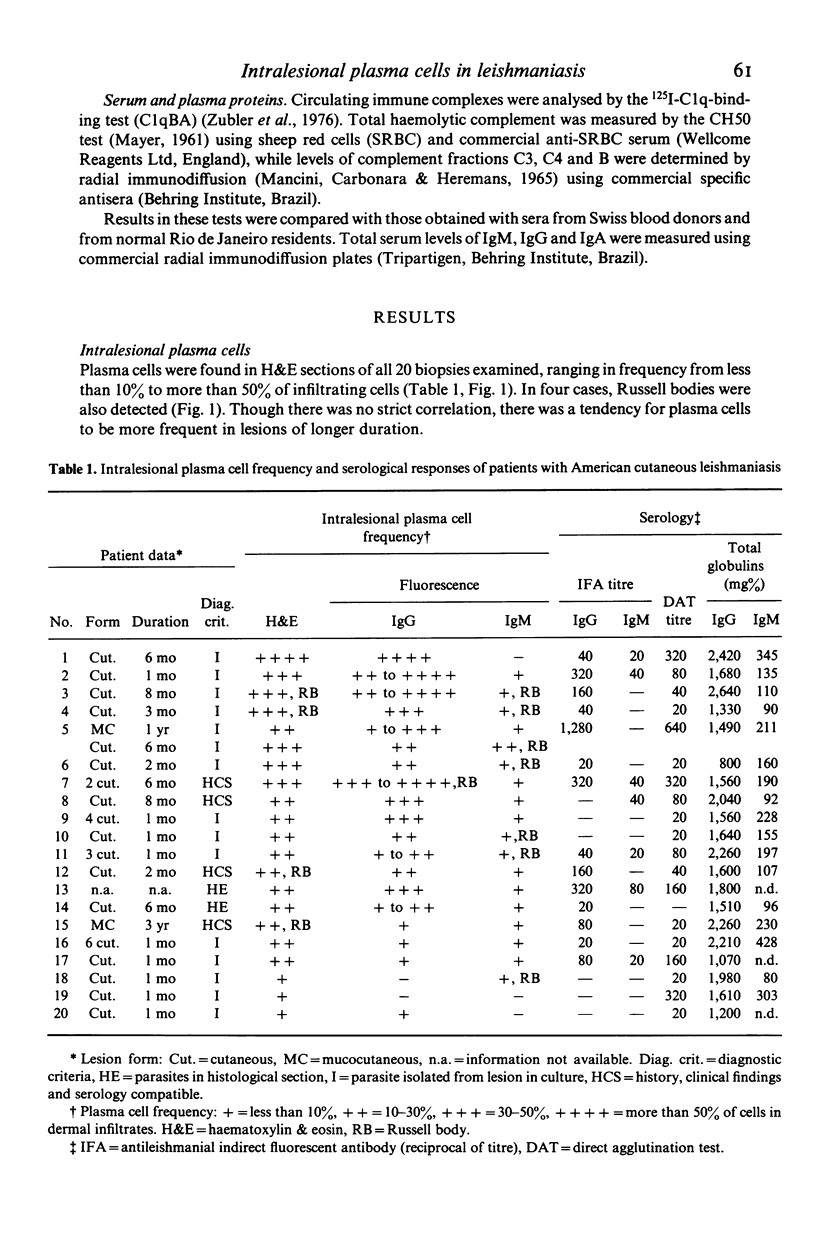
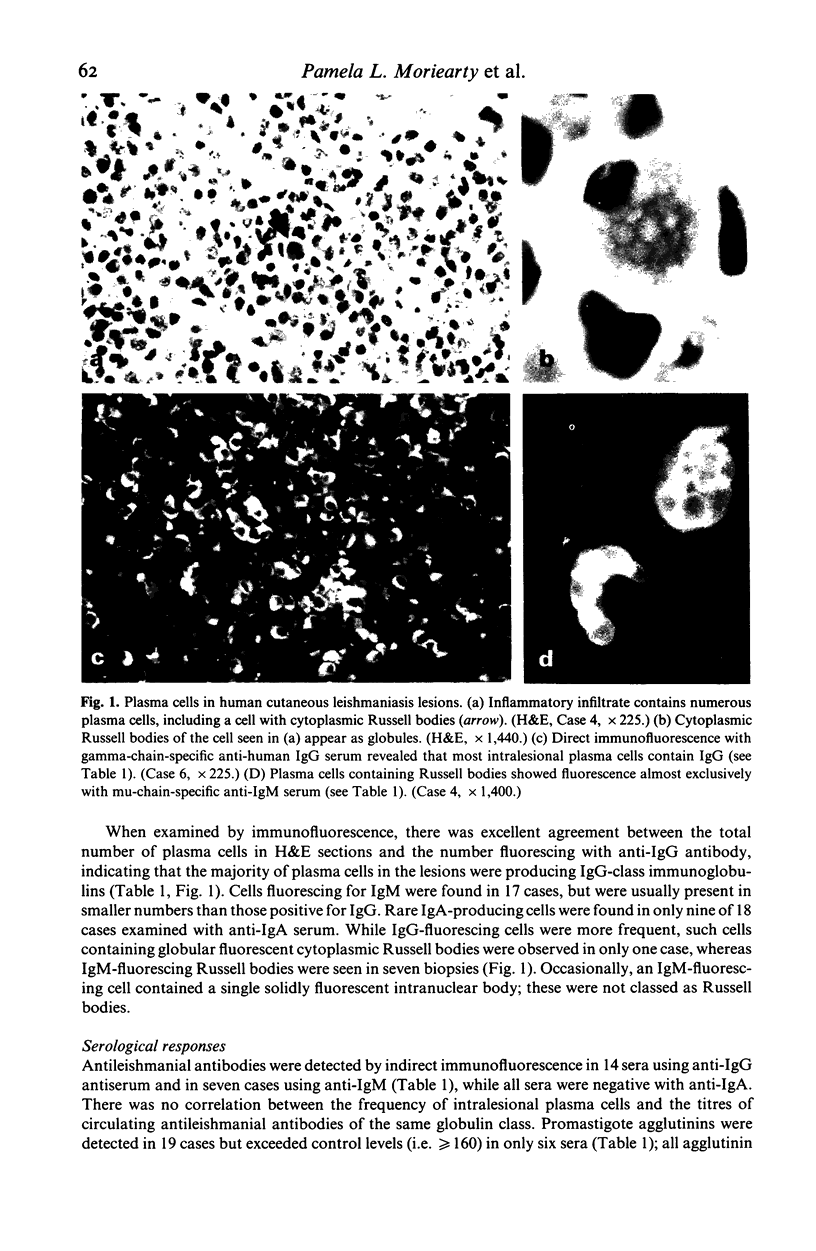
Intralesional plasma cells and serological responses were investigated in 20 Brazilian cases of cutaneous leishmaniasis. Plasma cell numbers varied from less than 10% to more than 50% of cells in inflammatory infiltrates, in general with greater numbers of such cells present in lesions of longer duration. Direct fluorescence examination with anti-IgG, -IgA and -IgM sera of trypsin-treated sections of formalin-fixed biopsy tissue revealed that most intralesional plasma cells contained IgG. Russell bodies were detected in eight cases, in seven of which these bodies fluoresced only with anti-IgM serum. There was no correlation between serum levels of total IgG, IgA and IgM (detected by radial immunodiffusion) or antileishmanial antibodies (detected by class-specific indirect immunofluorescence and by direct agglutination with and without 2-mercaptoethanol) and numbers of intralesional plasma cells of the same globulin class. No striking or consistent alterations in complement components were noted in the serum of these patients.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blom J., Mansa B., Wilk A. A study of Russell bodies in human monoclonal plasma cells by means of immunofluorescence and electron microscopy. Acta Pathol Microbiol Scand A. 1976 Jul;84(4):335–349. doi: 10.1111/j.1699-0463.1976.tb00124.x. [DOI] [PubMed] [Google Scholar]
- Grimaldi G., Jr, Moriearty P. L., Hoff R. Leishmania mexicana: immunology and histopathology in C3H mice. Exp Parasitol. 1980 Aug;50(1):45–56. doi: 10.1016/0014-4894(80)90006-5. [DOI] [PubMed] [Google Scholar]
- Huang S. N., Minassian H., More J. D. Application of immunofluorescent staining on paraffin sections improved by trypsin digestion. Lab Invest. 1976 Oct;35(4):383–390. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F. Responses to infection with metazoan and protozoan parasites in mice. Adv Immunol. 1979;28:451–511. doi: 10.1016/s0065-2776(08)60803-2. [DOI] [PubMed] [Google Scholar]
- Poulter L. W. Mechanisms of immunity to leishmaniasis. I. Evidence for a changing basis of protection in self-limiting disease. Clin Exp Immunol. 1980 Jan;39(1):14–26. [PMC free article] [PubMed] [Google Scholar]
- Preston P. M., Dumonde D. C. Experimental cutaneous leishmaniasis. V. Protective immunity in subclinical and self-healing infection in the mouse. Clin Exp Immunol. 1976 Jan;23(1):126–138. [PMC free article] [PubMed] [Google Scholar]
- Qualman S. J., Keren D. F. Immunofluorescence of deparaffinized, trypsin-treated renal tissues. Preservation of antigens as an adjunct to diagnosis of disease. Lab Invest. 1979 Dec;41(6):483–489. [PubMed] [Google Scholar]
- Ridley D. S. The pathogenesis of cutaneous leishmaniasis. Trans R Soc Trop Med Hyg. 1979;73(2):150–160. doi: 10.1016/0035-9203(79)90199-8. [DOI] [PubMed] [Google Scholar]
- Vattuone N. H., Yanovsky J. F. Trypanosoma cruzi: agglutination activity of enzyme-treated epimastigotes. Exp Parasitol. 1971 Dec;30(3):349–355. doi: 10.1016/0014-4894(71)90098-1. [DOI] [PubMed] [Google Scholar]
- Zubler R. H., Lange G., Lambert P. H., Miescher P. A. Detection of immune complexes in unheated sera by modified 125I-Clq binding test. Effect of heating on the binding of Clq by immune complexes and application of the test to systemic lupus erythematosus. J Immunol. 1976 Jan;116(1):232–235. [PubMed] [Google Scholar]