Abstract
Erythropoietin (EPO), originally identified for its critical hormonal role in promoting erythrocyte survival and differentiation, is a member of the large and diverse cytokine superfamily. Recent studies have identified multiple paracrine/autocrine functions of EPO that coordinate local responses to injury by maintaining vascular autoregulation and attenuating both primary (apoptotic) and secondary (inflammatory) causes of cell death. Experimental evidence also supports a role for EPO in repair and regeneration after brain and spinal cord injury, including the recruitment of stem cells into the region of damage. Tissue expression of the EPO receptor is widespread, especially during development, and includes the heart. However, it is currently unknown as to whether EPO plays a physiological function in adult myocardial tissue. We have assessed the potential protective role of EPO in vitro with adult rat cardiomyocytes, and in vivo in a rat model of myocardial infarction with reperfusion. The results show that EPO markedly prevents the apoptosis of cultured adult rat myocardiocytes subjected to 28 h of hypoxia (≈3% normal oxygen). Additional studies employing a rat model of coronary ischemia–reperfusion showed that the administration of recombinant human EPO (5,000 units/kg of body weight; i.p. daily for 7 days) reduces cardiomyocyte loss by ≈50%, an extent sufficient to normalize hemodynamic function within 1 week after reperfusion. These observations not only suggest a potential therapeutic role for recombinant human EPO in the treatment of myocardial ischemia and infarction by preventing apoptosis and attenuating postinfarct deterioration in hemodynamic function, but also predict that EPO is likely a tissue-protective cytokine in other organs as well.
The mortality and morbidity of ischemic cardiovascular disease remain the greatest unsolved public health challenge throughout the industrialized world. Although the technical advances of thrombolysis and angioplasty have resulted in a remarkable increase over the last 20 years in both the short- and long-term survival of patients reaching advanced medical care, a significant percentage of surviving patients remain severely disabled. Despite multiple significant therapeutic advances, ischemia/reperfusion (I/R) myocardial injury is still a major unsolved problem. Novel approaches that protect and salvage injured heart tissue would constitute important advances in the therapy of this disease.
In its classical (originally) recognized role, erythropoietin (EPO) and its receptor (EPOR) are indispensable for the survival, proliferation and differentiation of erythroid progenitor cells. From a broad perspective, EPO and EPOR function as the primary mediators of a general protective response to tissue hypoxia, which acts to maintain adequate tissue oxygenation through adjustments of circulating red cell mass by using a hormonal feedback-control system involving the kidney and the bone marrow (reviewed in ref. 1). More recently, it has been recognized that EPO and EPORs are also expressed by other tissues and organs, including the brain and heart (reviewed in ref. 2). The critical importance of these proteins is proven by knockout experiments targeting either the EPO or EPOR genes. Either genotype is an embryonic lethal because of erythrocyte aplasia with, however, major developmental abnormalities of the CNS (3) and the heart (4). EPO has also been shown to stimulate mitosis and signaling in astrocytes (5), endothelial cells (6), cardiomyoblasts (7), and cardiomyocytes (8) maintained in vitro.
With these observations as a rationale, studies have identified a major protective role for EPO in the brain, where it has been shown that locally administered recombinant human EPO (rhEPO) prevents ischemia-induced neuronal death (9, 10). Of more practical potential clinical utility, the same protection has also been observed after systemic administration of rhEPO, even when given after an ischemic insult. For example, rhEPO administered i.p. before or up to 6 h in the setting of focal brain ischemia in rats crosses the blood–brain barrier and reduces the severity of injury by 50–75% (11). Encouragingly, a successful translation from the rodent model to a proof-of-concept trial in human stroke has been recently reported (12). In other preclinical models, rhEPO also appears effective against a wide variety of other types of nervous system injuries that occur from inflammation (5). For example, within the setting of experimental autoimmune encephalitis (one model for multiple sclerosis), rhEPO markedly reduces the production and release of proinflammatory cytokines and chemokines, and dramatically reduces the influx of inflammatory cells into injured tissue (11, 13).
Other studies performed in the CNS have also identified critical vascular effects of rhEPO, which act to maintain adequate tissue perfusion, and, in this manner, markedly limit injury, such as the prevention of delayed ischemia after subarachnoid hemorrhage (14). These beneficial vascular effects are not limited to the brain, however, as rhEPO dramatically improves survival in a rat model of septic shock induced by splanchnic artery occlusion by means of a direct inhibition of inducible nitric oxide synthetase (iNOS) activity by peritoneal macrophages (15). A potential role for EPO in the heart to maintain tissue perfusion is of obvious significance.
The myocardium exhibits, in common with other tissues, including the brain, an endogenous protective system that results in an increased resistance to ischemic stress after brief episodes of nontoxic hypoxia. This phenomenon, termed preconditioning, has two distinct phases (reviewed in ref. 16). The first phase requires a few minutes of hypoxia and depends on a direct regulation of cation fluxes. Its protection is brief. The second phase is delayed (days), requires protein synthesis, and generally includes activation of hypoxia-inducible factor 1 (HIF-1) with subsequent up-regulation of hypoxia-sensitive genes that act to improve tissue performance under metabolic stress. Of importance is that one of the genes induced by HIF-1 is EPO. EPO has been proposed as being the mediator of preconditioning in the brain.
Like the brain injury, ischemic myocardial injury is characterized by a central region of cellular necrosis, surrounded by a variably sized penumbra or “volume at risk” (VAR) wherein cells typically undergo a delayed form of death. Recent evidence has clearly shown that a substantial portion of the cardiomyocyte loss after myocardial infarction and reperfusion arises from apoptosis within this region (17, 18). Additionally, a significant proportion of the delayed form of injury occurs as a result of recruitment of inflammatory cells into the infarcted region, which then release chemotactic and cytotoxic cytokines and other inflammatory molecules expanding the volume of injury in an amplifying positive-feedback loop.
Encouraged by the substantial body of evidence demonstrating sizeable beneficial effects of EPO in models of nervous-system injury with a similar pathophysiology to cardiac ischemia, we directly determined whether rhEPO modifies hypoxia-induced apoptosis of cultured adult rat cardiomyocytes. These experiments confirmed a direct antiapoptotic effect of rhEPO. Additional study used a rat model of cardiac I/R injury. The results show that myocardiocyte losses and the resulting hemodynamic dysfunction produced by 30 min of occlusion of the left descending coronary artery are largely prevented by the administration of rhEPO. We selected a model of I/R for study rather than one of permanent ischemia, because it closely mimics the clinical situation of an acute myocardial infarction with early reperfusion, producing a necrotic core surrounded by a substantial VAR.
Materials and Methods
All procedures involving animals were conducted in conformity with institutional guidelines in compliance with national and international laws and policies (19–22). Clinical grade rhEPO was obtained from OrthoBiotech (Raritan, NJ) and other reagents were from local suppliers unless otherwise specified.
In Vitro Protocol.
Left ventricular cardiomyocytes were isolated from 3-month-old male Sprague–Dawley rats as described (23, 24). Briefly, under chloral hydrate anesthesia (300 mg/kg of body weight), hearts were excised, and the myocytes were dissociated by collagenase. Cardiomyocytes (98–99% pure) were plated onto Petri dishes coated with 0.5 μg/cm2 laminin at a density of 2 × 104 cells per cm2. Cells were incubated in serum-free medium consisting of modified Eagle's medium (MEM) with nonessential amino acids, transferrin (10 μg/ml), BSA (0.1%), and antibiotics. To remove unattached myocytes, this medium was exchanged for new medium 30 min after plating. Hypoxic conditions were generated by exposure in an air-tight chamber flushed continuously with nitrogen and maintained for 28 h. This procedure reduced oxygen tension in the medium to 5 mmHg (≈3% normal; 1 mmHg = 133 Pa). Hypoxia was maintained for 28 h. Where indicated, rhEPO (100 ng/ml), Hepes (20 mM), or both were added to the medium 30 min before the induction of hypoxia. Hepes was used to correct the acidosis produced by prolonged hypoxia.
Cellular necrosis was quantified by using two independent methods (ethidium monoazide bromide; Molecular Probes) and hairpin oligonucleotide probe with blunt ends (hairpin 2; Synthetic Genetics, San Diego). Apoptosis was assessed by use of a hairpin oligonucleotide probe with single-base 3′ overhangs (hairpin 1; Synthetic Genetics) and a terminal deoxynucleotidyltransferase (TdT) assay (25). The number of myocytes measured in each preparation varied from a minimum of 300 to a maximum of 800. Results are presented as mean ± SD. The significance of differences between two measurements was determined by using Student's t test and that for multiple comparisons, by ANOVA and the Bonferroni method. P values of <0.05 were considered significant. In all cases, replicate values correspond to additional independent cell isolations.
In Vivo Experimental Protocol.
Male Sprague–Dawley rats (253 ± 4 g) were anesthetized with chloral hydrate (150 mg/kg i.p.) and diethyl ether and were ventilated (61 breaths per min, tidal volume 1.2 ml/100 g of body weight) through an endotracheal cannula. The left anterior descending coronary artery (LAD) was ligated with a 5-S silk suture after exteriorization of the heart through a 15-mm opening at the fifth intercostal space. A plain knot was tied over two pieces of suture, which was removed after 30 min to initiate reperfusion. The thorax was closed under negative pressure, and the rat was weaned from mechanical ventilation under continuous electrocardiographic monitoring. Ischemia was confirmed by the appearance of ventricular ectopy and blanching of the myocardium. Successful reperfusion was indicated by a restoration of normal rubor. Sham-operated rats underwent identical surgical procedures, but without ligation of the LAD. rhEPO was administered i.p. in all experiments at the same dose of 5,000 units/kg of body weight, either as two doses 24 h and 0.5 h before the induction of ischemia (pretreatment) or as a single dose at reperfusion (posttreatment). Each animal received an additional dose of rhEPO daily until study completion. rhEPO repeatedly administered i.p. was not associated with any evident adverse consequences. However, the hematocrit increased over 7 days from baseline 44 ± 2% to 55 ± 1% (P < 0.01) in these non-iron-supplemented rats.
To determine the extent of myocardial infarction and surrounding VAR, the chest was reopened after 4 h of reperfusion and the VAR was determined by retightening the ligature around the coronary artery and injecting 2 ml of 5% Evans blue in water into the right ventricular chamber. Myocardium outside the occluded vessel stained blue, whereas the VAR remained unstained. The heart was arrested by 0.5 M KCl injection, removed from the chest, and processed in two different ways, depending on the assessment to be performed. For determination of infarct volume by tetrazolium salt reduction [triphenyltetrazolium chloride (TTC); 1% in saline], the parts unstained with Evans blue were separated and incubated for 20 min at 20°C in TTC, then were transferred in 4% buffered formalin. The area of perfused tissue (blue), viable ischemic tissue (red), and dead ischemic tissue (white) was measured by using an automatic analyzer (Analytical Imaging Station, Version 3.0, Imaging Research, St. Catherine's, ON, Canada). The total volume of the different parts was then computed.
Hemodynamic Measurements.
Rats surviving a 30-min LAD occlusion for 7 days underwent hemodynamic evaluation followed by fixation perfusion of the heart for histomorphometry. Under pentobarbital anesthesia (50 mg/kg i.p.), a microtip pressure transducer (SPC-320, Millar Instruments, Houston) connected to an amplifier and recorder (Windowgraph, Gould Electronics, Valley View, OH) was inserted into the right carotid artery to measure systolic and diastolic blood pressure, and heart rate. The pressure transducer was then advanced into the left ventricle (LV) to measure LV systolic (LVSP) and LV end-diastolic pressure (LVEDP), the first derivatives (positive and negative) of LV pressure over time.
On completion of hemodynamic measurements, a PE-200 cannula was inserted in the abdominal aorta, and the heart was arrested in diastole by injection of a bolus of 4 ml of 0.5 M KCl into the aorta and was perfused for 3 min with Ca- and Mg-free PBS with 10 units/ml heparin. Finally, the heart was perfused with phosphate-buffered 4% formaldehyde for 30 min at the LVEDP previously measured so as to maintain the appropriate ventricular volume.
These specimens were fixed for an additional 24 h and were then examined histologically as described (26). Briefly, the atria were trimmed and the free right ventricle (RV) wall and LV inclusive of the septum were weighed individually. LV internal length was measured by use of a probe. A slice of ≈3 mm obtained from immediately below the LAD ligature was used for measuring wall thickness and chamber area to determine LV geometry and, by application of La Place's law, wall stress. Two sections from each heart were obtained (each was 5 μm thick; one just below the ligature, the other from the basal side of the apical slice) and were stained with hematoxylin/eosin, and the infarct area was calculated by direct cardiomyocyte-nuclei counting. The cardiomyocyte cross-sectional area was measured by image analyzer (IBAS 2.0, Kontron-Zeiss Image Analysis System) at ×400 and averaged over 50 cardiomyocytes in each section. Measurements obtained in the two representative sections of each heart were averaged. Results are presented as mean ± SEM. These data were evaluated by using ANOVA and analysis of covariance (ANCOVA) with treatment and myocardial infarct size as covariants using jmp (SAS Insitute, Cary, NC).
Results
Isolated Cardiomyocytes.
After 28 h of hypoxia, ≈60% of untreated myocardiocytes were nonviable. Of these, ≈85% were apoptotic (Fig. 1 A and B) and the remaining small fraction were necrotic (Fig. 1 C and D). In the presence of 100 ng/ml rhEPO, apoptosis was reduced by half. In contrast, rhEPO exposure did not affect the number of necrotic cells observed. Normalization of the medium pH to 7.6 by Hepes buffering reduced the overall cell death slightly, but did not affect the ability of rhEPO to rescue a significant number of cells from apoptosis (Fig. 2 and Fig. 5, which is published as supporting information on the PNAS web site, www.pnas.org), nor did it change the proportion of cells undergoing apoptosis.
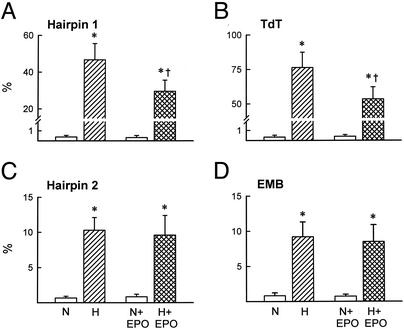
Figure 1.
(A and B) Inclusion of rhEPO (100 ng/ml) in the culture medium of adult rat cardiomyocytes reduced cell apoptosis in the presence of acidosis. (C and D) In contrast, the presence of rhEPO did not reduce necrosis, which was a smaller component of cell death. EMB, ethidium monoazide bromide; TdT, terminal deoxynucleotidyltransferase; N, normoxia; H, hypoxia. Results are means ± SD; n = 5 or 6 in each determination. *, P < 0.05 vs. N or N + EPO; †, P < 0.05 vs. H.
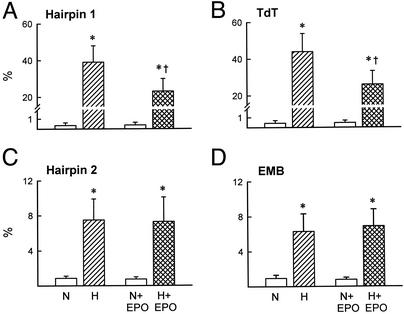
Figure 2.
Correction of acidosis by using Hepes did not reduce the antiapoptotic action of rhEPO in vitro. Abbreviations are as in Fig. 1. Results are means ± SD; n = 5 or 6 in each determination. *, P < 0.05 vs. N, or N + EPO, and H + EPO; †, P < 0.05 vs. H.
In Vivo Experiments.
Thirty minutes of reversible occlusion of the LAD produced a moderately large infarction of the LV (mean 18 ± 7.5% of LV mass; range 5–42%; n = 72). During the early (4-h) reperfusion period, mortality was ≈25% for both groups (vehicle; 4/24 and rhEPO; 7/31). The VAR, as defined by the exclusion of Evans blue dye, was also similar for both groups, at 48.5% and 45.5% of LV weight for vehicle and rhEPO, respectively. Prior exposure to rhEPO did not significantly affect the extent of tetrazolium staining observed (vehicle = 35% and rhEPO = 38% of ventricular mass).
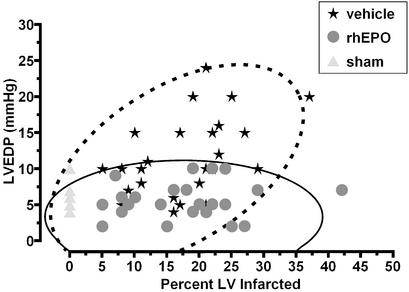
After 7 days of reperfusion, the saline-treated group exhibited a hemodynamic deterioration characterized by increased LVEDP and myocardial wall stress (Figs. 3 and 4). The degree of impairment was related to the infarct size in only the saline-treated group, for which, as expected, larger infarcts were associated with greater dysfunction (Fig. 3). In contrast, no relationship between infarct size and dysfunction was noted, and further, no animal receiving rhEPO exhibited a clearly abnormal LVEDP.
Figure 3.
Correlation between LVEDP and infarct size after 7 days of reperfusion. Animals administered vehicle alone exhibited an increase in LVEDP as a function of increasing infarct size. In contrast, LVEDP of animals receiving rhEPO was unrelated to infarct size. Bivariate normal distribution of LVEDP vs. infarct size at the 95% level is indicated by the ellipses, saline treatment by the dashed lines, and rhEPO by the solid lines, respectively.
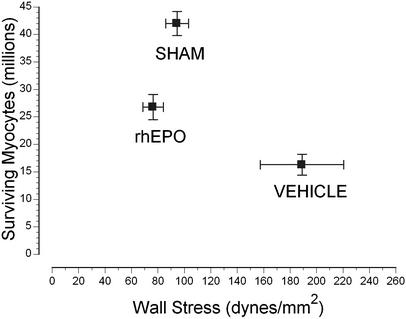
Figure 4.
LV cardiomyocyte number loss was attenuated by rhEPO treatment. Despite a significant loss, ventricular wall stress remained normal. In contrast, vehicle-treated animals exhibited marked increases in wall stress. Error bars are SEM.
A crucial underlying difference between the rhEPO and vehicle groups was clarified by quantification of surviving cardiomyocytes. Specifically, the number of cardiomyocyte nuclei in the LV was decreased by >60% in I/R vehicle group compared with the sham group (P < 0.01). In contrast, within the rhEPO group, cardiomyocyte nuclei were reduced by only 35% (P < 0.01 vs. vehicle). Despite the significant loss of cardiomyocytes in the rhEPO group, wall stress was not increased above normal (Fig. 4). Similarly, cardiomyocyte volume normalized for nuclear density increased nearly 3-fold in the vehicle group (15.6 ± 0.5 × 103 μm3 in sham to 45.0 ± 0.6 × 103 μm3 in vehicle) but less than twice (27.0 ± 0.2 × 103 μm3; P < 0.05 vs. vehicle) in the rhEPO group. Direct estimation of cardiomyocyte cross-sectional area also showed similar trends to the histomorphometric information (data not shown).
To address the importance of the pretreatment doses of rhEPO in this model, we omitted rhEPO pretreatment before coronary occlusion and began giving rhEPO immediately after reperfusion began. The 7-day mortality [5/15 (33%) and 4/15 (27%)] and infarct size (18 ± 1% of the LV) were unchanged in the control and rhEPO groups. As in the pretreatment group, rhEPO exposure was associated with normalized LVEDP 1 week after infarction (10.4 ± 1.0 mmHg in placebo; n = 10, and 4.7 ± 1.0 mmHg in EPO; n = 11; P < 0.0001) as well as a near-normal estimated ventricular wall stress.
Discussion
The observations obtained from the in vitro experiments directly demonstrate that rhEPO prevents apoptosis of myocardial cells exposed to extreme and prolonged hypoxia. In this model of cardiac injury, reduction of medium oxygen content to <1% triggers a majority of cells to enter into apoptosis that can be prevented by rhEPO exposure. In contrast, relatively few cardiomyocytes undergo necrosis, and these do not respond to rhEPO. The observation of an involvement of rhEPO only within the apoptosis group is similar to findings obtained from the nervous system (11, 27–29).
I/R within the myocardium is well known to result in cardiomyocyte dysfunction and death by multiple pathways. Previous studies have shown that a significant dilatation of the LV begins immediately after coronary occlusion in rats, producing increases in LVEDP and a diminished slope of LV pressure vs. time (30, 31). These alterations markedly increase myocardial wall stress. It is now clear that the cytoarchitecture of the myocardium is not strictly fixed, but that it reorganizes under mechanical stress by undergoing a side-to-side slippage of cardiomyocytes with respect to each other (32, 33). This process produces a gradual, often eccentric, increase of ventricular volume. Although these changes typically constitute a beneficial adaptation during the acute phase of a cardiac infarction by maintaining critical pump function, such remodeling necessarily results in inefficient pump function that leads, in turn, to gradual hemodynamic deterioration. Additionally, the cost of increased work required by an abnormally high myocardial wall tension is often amplified by a further decreased perfusion of oxygen and nutrients, coupled with a compensatory increase in heart rate in an attempt to maintain adequate cardiac output. Because this work must be performed by a reduced number of myocardiocytes, continued work-related injury tends to reduce even further the number of adequately functioning cardiomyocytes by triggering apoptosis (34).
It is notable that we failed to observe any significant changes in acute infarct volume (at 4 h) by use of two histomorphic methods, but we nonetheless observed large histological differences and significant functional outcomes by 1 week after reperfusion. This finding is likely explained by a continued attrition of myocardiocytes in the days after infarction in the vehicle-treated animals: presumably cells within the VAR continue to undergo apoptosis as a result of work-induced injury over an extended period. Whether the action of rhEPO is primarily explained by an increased cell survival or improved myocardiocyte function (or both) is currently unclear.
The molecular signals by which rhEPO provides its benefit in these models is currently unclear. Recent data obtained from study of the nervous system have shown that rhEPO signals through the serine/threonine kinase Akt, which is also activated by other ligands (27). Akt signaling, e.g., by insulin (35), or constitutive activation (37), reduces I/R injury of the rat heart, as assessed by cell loss and hemodynamic function. In the nervous system, Akt activation ultimately results in up-regulation of the bcl family of antiapoptotic genes. NF-κB, a key mediator of inflammatory and cytokine responses, has also been implicated in rhEPO signaling (33) and plays a role in preconditioning as well (16). Relevance of these separate pathways in the response of the myocardium to reperfusion injury has been clearly established, and pharmacological manipulation of each has been shown to improve outcome after ischemia and infarction (38, 39). Further study is required to understand the role of EPO of these (and other) adaptive responses in more detail.
Several limitations to conclusions drawn from these studies warrant noting. First, several critical variables were calculated from morphometric data obtained after death and these invariably involve inaccuracies. Second, it has been shown that for the calculation of infarct size, the fractional area occupied by the scar is also subject to errors because of scar shrinkage, infarct expansion, and reactive hypertrophy of spared myocardium. Although we believe the factors to contribute only small errors, to augment these data we also assessed infarct size by calculating the actual loss of cardiomyocytes. These calculations showed that not only does rhEPO reduce cardiomyocyte loss over 7 days, but it also attenuates the reactive hypertrophy of surviving cardiomyocytes. A greater number of spared myocytes, together with their smaller size, could well explain the remarkable improvement observed in cardiac function. Third, a daily dose of 5,000 units/kg of body weight of rhEPO was sufficient to increase hematocrit by 20–30% by day 7, and such a change in erythrocyte mass might improve cardiac function merely by improving delivery of oxygen to a hypoxic myocardium. This conclusion seems unlikely for a several reasons. Most importantly, this model utilizes young animals without underlying cardiovascular abnormalities. Thus, on reperfusion, it is not likely that significant myocardium will be characterized by decreased perfusion, and therefore any practical limitation in oxygen delivery. On the other hand, an increase in hematocrit may itself tend to increase hypoxia through adverse rheological effects. Very recently, it has been shown in a transgenic mouse model that increased EPO concentrations lead to a profound alteration of the regulation of vascular tone secondary to changes within the opposing nitric oxide and endothelin vasoactive systems (40). However, under conditions of a lower-than-normal erythrocyte mass, changes in hemoglobin concentration affected by rhEPO would likely be important. For example, a recent retrospective analysis of patients recovering from an acute myocardial infarction has shown that blood transfusion in patients with hematocrit <30% decreased 30-day mortality (41). Even more impressively, prospective studies have shown significant functional improvement in chronic-heart-failure patients treated with rhEPO (42). In these studies, however, the presence of a direct effect of rhEPO on the myocardium as independent from its effect in the bone marrow is not directly assessable. In view of the results observed in this model, a direct effect on the myocardium certainly must be entertained.
The beneficial effects observed in the in vivo model appear to be independent of the presence of rhEPO at the onset of cardiac ischemia, suggesting that these develop during reperfusion of previously ischemic myocardium. It is therefore unlikely that rhEPO acts through a short-latency-type preconditioning, which would reduce infarct size. The lack of effect indirectly suggests that NF-κB, an important mediator of ischemic acute preconditioning, may not be a target of rhEPO in the myocardium. These data also suggest that the effects of rhEPO may be even more impressive in the setting of larger infarcts (>30%; Fig. 5), especially when assessed beyond the stage of completed scar formation (3–4 weeks). Finally, a potential role for rhEPO in the recruitment of stem cells into the region of injury, as has been recently observed for the nervous system (43), is an intriguing possibility.
In conclusion, the preservation by rhEPO of a normal cardiac hemodynamic function by 7 days after infarct rhEPO treatment can be explained, at least in part, by an attenuation of cardiomyocyte loss within the VAR. The protective effect of rhEPO is consistent with studies of isolated cardiomyocytes: fewer cells die during the course of experimental myocardial infarction, which leads to an attenuated hypertrophic compensatory response. These benefits do not appear to require pretreatment with rhEPO and, thus, are not likely to include a functional equivalent of acute preconditioning. Although the effects of rhEPO on erythrocyte mass are of minor concern in these acute experiments, this would not be true if rhEPO is used clinically in a chronic dosage regimen, e.g., for progressive heart failure. Development of nonerythropoietic analogues of EPO might allow researchers to avoid this potential adverse effect for testing in chronic-disease models. The significant effect of rhEPO within the injured heart also strongly supports the concept that EPO is possibly a tissue-protective cytokine for other organs that express the EPO receptor.
Supplementary Material
Acknowledgments
We thank Serge Masson and Fabio Fiordaliso for their expert advice and assistance.
Abbreviations
- EPO
erythropoietin
- EPOR
EPO receptor
- rhEPO
recombinant human EPO
- I/R
ischemia/reperfusion
- LAD
left anterior descending coronary artery
- LV
left ventricle
- LVEDP
LV-end diastolic pressure
- VAR
volume at risk
References
- 1.Jelkmann W, Metzen E. Anat Anz. 1996;178:391–403. doi: 10.1016/S0940-9602(96)80124-5. [DOI] [PubMed] [Google Scholar]
- 2.Masuda S, Nagao M, Sasaki R. Int J Hematol. 1999;70:1–6. [PubMed] [Google Scholar]
- 3.Yu X, Shacka J J, Eells J B, Suarez-Quian C, Przygodzki R M, Beleslin-Cokic B, Lin C S, Nikodem V M, Hempstead B, Flanders K C, et al. Development (Cambridge, UK) 2002;129:505–516. doi: 10.1242/dev.129.2.505. [DOI] [PubMed] [Google Scholar]
- 4.Wu H, Lee S H, Gao J, Liu X, Iruela-Arispe M L. Development (Cambridge, UK) 1999;126:3597–3605. doi: 10.1242/dev.126.16.3597. [DOI] [PubMed] [Google Scholar]
- 5.Sugawa M, Sakurai Y, Ishikawa-Ieda Y, Suzuki H, Asou H. Neurosci Res (NY) 2002;44:391–403. doi: 10.1016/s0168-0102(02)00161-x. [DOI] [PubMed] [Google Scholar]
- 6.Jaquet K, Krause K, Tawakol-Khodai M, Geidel S, Kuck K H. Microvasc Res. 2002;64:326–333. doi: 10.1006/mvre.2002.2426. [DOI] [PubMed] [Google Scholar]
- 7.Ogilvie M, Yu X, Nicolas-Metral V, Pulido S M, Liu C, Ruegg U T, Noguchi C T. J Biol Chem. 2000;275:39754–39761. doi: 10.1074/jbc.M004999200. [DOI] [PubMed] [Google Scholar]
- 8.Wald M R, Borda E S, Sterin-Borda L. J Cell Physiol. 1996;167:461–468. doi: 10.1002/(SICI)1097-4652(199606)167:3<461::AID-JCP10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Sadamoto Y, Igase K, Sakanaka M, Sato K, Otsuka H, Sakaki S, Masuda S, Sasaki R. Biochem Biophys Res Commun. 1998;253:26–32. doi: 10.1006/bbrc.1998.9748. [DOI] [PubMed] [Google Scholar]
- 10.Bernaudin M, Marti H H, Roussel S, Divoux D, Nouvelot A, MacKenzie E T, Petit E. J Cereb Blood Flow Metab. 1999;19:643–651. doi: 10.1097/00004647-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Brines M L, Ghezzi P, Keenan S, Agnello D, de Lanerolle N C, Cerami C, Itri L M, Cerami A. Proc Natl Acad Sci USA. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M, Rustenbeck H H, Breiter N, Jacob S, Knerlich F, et al. Mol Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- 13.Agnello D, Bigini P, Villa P, Mennini T, Cerami A, Brines M, Ghezzi P. Brain Res. 2002;952:128–134. doi: 10.1016/s0006-8993(02)03239-0. [DOI] [PubMed] [Google Scholar]
- 14.Grasso G, Buemi M, Alafaci C, Sfacteria A, Passalacqua M, Sturiale A, Calapai G, De Vico G, Piedimonte G, Salpietro F M, Tomasello F. Proc Natl Acad Sci USA. 2002;99:5627–5631. doi: 10.1073/pnas.082097299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Squadrito F, Altavilla D, Squadrito G, Campo G M, Arlotta M, Quartarone C, Saitta A, Caputi A P. Br J Pharmacol. 1999;127:482–488. doi: 10.1038/sj.bjp.0702521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nandagopal K, Dawson T M, Dawson V L. J Pharmacol Exp Ther. 2001;297:474–478. [PubMed] [Google Scholar]
- 17.Anversa P, Cheng W, Liu Y, Leri A, Redaelli G, Kajstura J. Basic Res Cardiol. 1998;93, Suppl. 3:8–12. doi: 10.1007/s003950050195. [DOI] [PubMed] [Google Scholar]
- 18.Gottlieb R A, Burleson K O, Kloner R A, Babior B M, Engler R L. J Clin Invest. 1994;94:1621–1628. doi: 10.1172/JCI117504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Law by Decree No. 116 (February 18, 1992) Gazzetta Ufficiale della Repubblica Italiana, Suppl. 40.
- 20. Circular No. 8 (July 14, 1994) Gazzetta Ufficiale della Repubblica Italiana.
- 21. European Economic Council Directive 86/609 (December 12, 1987) Official Journal of Law, p. 358.
- 22.Committee on Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. Washington, DC: Natl. Acad. Press; 1996. [Google Scholar]
- 23.Fiordaliso F, Leri A, Cesselli D, Limana F, Safai B, Nadal-Ginard B, Anversa P, Kajstura J. Diabetes. 2001;50:2363–2375. doi: 10.2337/diabetes.50.10.2363. [DOI] [PubMed] [Google Scholar]
- 24.Leri A, Claudio P P, Li Q, Wang X, Reiss K, Wang S, Malhotra A, Kajstura J, Anversa P. J Clin Invest. 1998;101:1326–1342. doi: 10.1172/JCI316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cigola E, Kajstura J, Li B, Meggs L G, Anversa P. Exp Cell Res. 1997;231:363–371. doi: 10.1006/excr.1997.3477. [DOI] [PubMed] [Google Scholar]
- 26.Anversa P, Capasso J M, Puntillo E, Sonnenblick E H, Olivetti G. Pathol Res Pract. 1989;185:544–550. doi: 10.1016/S0344-0338(89)80190-6. [DOI] [PubMed] [Google Scholar]
- 27.Siren A L, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, Keenan S, Gleiter C, Pasquali C, Capobianco A, et al. Proc Natl Acad Sci USA. 2001;98:4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Celik M, Gokmen N, Erbayraktar S, Akhisaroglu M, Konakc S, Ulukus C, Genc S, Genc K, Sagiroglu E, Cerami A, Brines M. Proc Natl Acad Sci USA. 2002;99:2258–2263. doi: 10.1073/pnas.042693799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juul S E, Anderson D K, Li Y, Christensen R D. Pediatr Res. 1998;43:40–49. doi: 10.1203/00006450-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Pfeffer J M, Pfeffer M A, Fletcher P J, Braunwald E. Am J Physiol. 1991;260:H1406–H1414. doi: 10.1152/ajpheart.1991.260.5.H1406. [DOI] [PubMed] [Google Scholar]
- 31.Pfeffer J M, Pfeffer M A, Fletcher P J, Braunwald E. Am J Med. 1984;76:99–103. doi: 10.1016/0002-9343(84)90894-5. [DOI] [PubMed] [Google Scholar]
- 32.Olivetti G, Quaini F, Lagrasta C, Cigola E, Ricci R, Maestri R, Anversa P. Cardioscience. 1995;6:101–106. [PubMed] [Google Scholar]
- 33.Olivetti G, Capasso J M, Sonnenblick E H, Anversa P. Circ Res. 1990;67:23–34. doi: 10.1161/01.res.67.1.23. [DOI] [PubMed] [Google Scholar]
- 34.Cheng W, Li B, Kajstura J, Li P, Wolin M S, Sonnenblick E H, Hintze T H, Olivetti G, Anversa P. J Clin Invest. 1995;96:2247–2259. doi: 10.1172/JCI118280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jonassen A K, Sack M N, Mjos O D, Yellon D M. Circ Res. 2001;89:1191–1198. doi: 10.1161/hh2401.101385. [DOI] [PubMed] [Google Scholar]
- 36.Matsui T, Tao J, del Monte F, Lee K H, Li L, Picard M, Force T L, Franke T F, Hajjar R J, Rosenzweig A. Circulation. 2001;104:330–335. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- 37.Digicaylioglu M, Lipton S A. Nature. 2001;412:641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- 38.Thourani V H, Brar S S, Kennedy T P, Thornton L R, Watts J A, Ronson R S, Zhao Z Q, Sturrock A L, Hoidal J R, Vinten-Johansen J. Am J Physiol. 2000;278:H2084–H2093. doi: 10.1152/ajpheart.2000.278.6.H2084. [DOI] [PubMed] [Google Scholar]
- 39.Ritchie M E. Circulation. 1998;98:1707–1713. doi: 10.1161/01.cir.98.17.1707. [DOI] [PubMed] [Google Scholar]
- 40. Quaschning, T., Ruschitzka, F., Stallmach, T., Shaw, S., Morawietz, H., Goettsch, W., Hermann, M., Slowinski, T., Theuring, F., Hocher, B., et al. (2003) FASEB J. 17, 259–261. [DOI] [PubMed]
- 41.Wu W C, Rathore S S, Wang Y, Radford M J, Krumholz H M. N Engl J Med. 2001;345:1230–1236. doi: 10.1056/NEJMoa010615. [DOI] [PubMed] [Google Scholar]
- 42.Silverberg D S, Wexler D, Sheps D, Blum M, Keren G, Baruch R, Schwartz D, Yachnin T, Steinbruch S, Shapira I, Laniado S, Iaina A. J Am Coll Cardiol. 2001;37:1775–1780. doi: 10.1016/s0735-1097(01)01248-7. [DOI] [PubMed] [Google Scholar]
- 43.Shingo T, Sorokan S T, Shimazaki T, Weiss S. J Neurosci. 2001;21:9733–9743. doi: 10.1523/JNEUROSCI.21-24-09733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.