Abstract
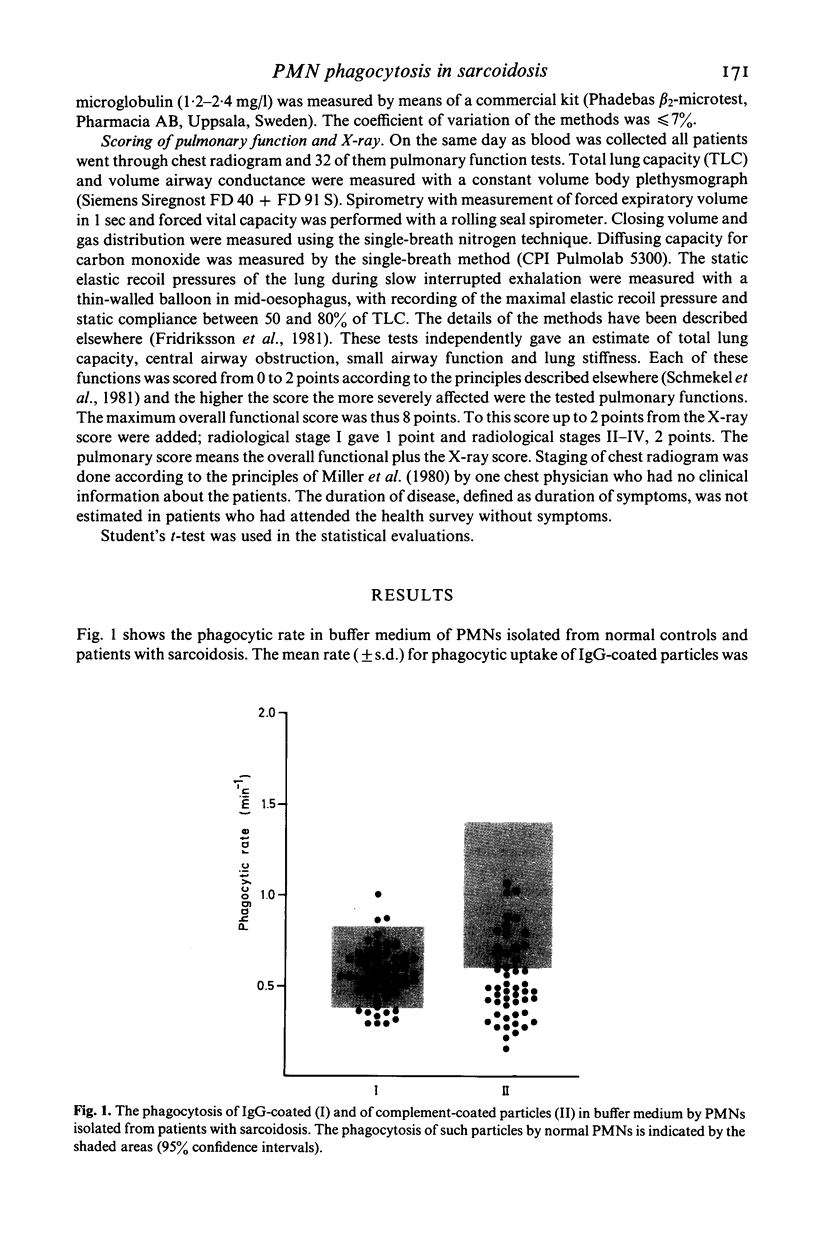
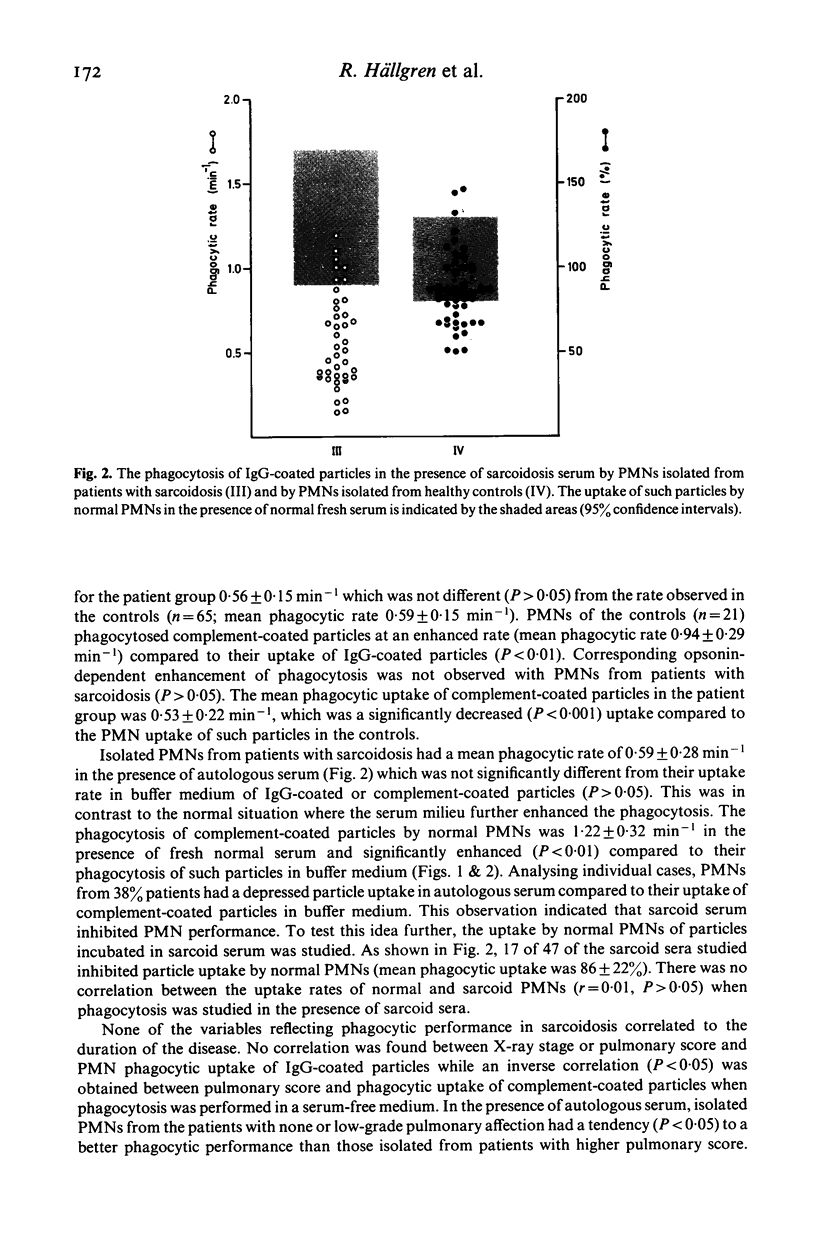
Kinetic measurements of the serum-independent uptake of IgG-coated or complement-opsonized latex particles have been performed in 58 patients with sarcoidosis. The mean rate for phagocytic uptake of IgG particles was 0·56 min-1 which was not different from that of the controls (0·59 min-1). The phagocytosis of complement-opsonized particles was in the patient group 0·53 min-1 and significantly (P<0·001) reduced compared to the rate of the controls (mean rate 0·94 min-1), indicating neutrophil C3b-receptor dysfunction in sarcoidosis. PMNs from patients with sarcoidosis were not stimulated by the presence of autologous serum in contrast to PMNs from normals and in individual cases even a reduced uptake was found. More than one-third of the sarcoid sera also inhibited the phagocytosis of normal PMNs indicating the presence of a phagocytosis-inhibitory activity in sarcoid sera. Patients with more severe lung affection as estimated by measurements of total lung capacity, central airway obstruction, small airway function and pulmonary X-ray changes had a more reduced PMN phagocytosis in the presence of autologous serum than those with minor signs of lung affection (P<0·05). The phagocytosis-inhibitory activity of sarcoid serum was also more pronounced in those individuals who had high pulmonary score (P<0·05) or radiographic stage II-IV sarcoidosis (P<0·01). No correlation was found between serum levels of lactoferrin or lysozyme and any of the phagocytic variables while elevated β2-microglobulin levels were associated with more pronounced serum-mediated inhibition of PMN phagocytosis (P<0·05). The relevance of these findings to the pathogenesis of granuloma formation in sarcoidosis is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ehlenberger A. G., Nussenzweig V. The role of membrane receptors for C3b and C3d in phagocytosis. J Exp Med. 1977 Feb 1;145(2):357–371. doi: 10.1084/jem.145.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gange R. W., Black M. M., Carrington P., McKerron R. Defective neutrophil migration in sarcoidosis. Lancet. 1977 Aug 20;2(8034):379–381. doi: 10.1016/s0140-6736(77)90306-3. [DOI] [PubMed] [Google Scholar]
- Hansen N. E., Malmquist J., Thorell J. Plasma myeloperoxidase and lactoferrin measured by radioimmunoassay: relations to neutrophil kinetics. Acta Med Scand. 1975 Dec;198(6):437–443. doi: 10.1111/j.0954-6820.1975.tb19572.x. [DOI] [PubMed] [Google Scholar]
- Hällgren R., Jansson L., Venge P. Kinetic studies of phagocytosis of IgG-coated latex particles with a thrombocyte counter. J Lab Clin Med. 1977 Nov;90(5):786–795. [PubMed] [Google Scholar]
- Håkansson L., Hällgren R., Venge P. Effect of hyaluronic acid on phagocytosis of opsonized latex particles. Scand J Immunol. 1980;11(6):649–653. doi: 10.1111/j.1365-3083.1980.tb00034.x. [DOI] [PubMed] [Google Scholar]
- Jaffe C. J., Vierling J. M., Jones E. A., Lawley T. J., Frank M. M. Receptor specific clearance by the reticuloendothelial system in chronic liver diseases. Demonstration of defective C3b-specific clearance in primary biliary cirrhosis. J Clin Invest. 1978 Nov;62(5):1069–1077. doi: 10.1172/JCI109212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maderazo E. G., Ward P. A., Woronick C. L., Kubik J., DeGraff A. C., Jr Leukotactic dystunction in sarcoidosis. Ann Intern Med. 1976 Apr;84(4):414–419. doi: 10.7326/0003-4819-84-4-414. [DOI] [PubMed] [Google Scholar]
- Mantovani B. Different roles of IgG and complement receptors in phagocytosis by polymorphonuclear leukocytes. J Immunol. 1975 Jul;115(1):15–17. [PubMed] [Google Scholar]
- Pascual R. S., Gee J. B., Finch S. C. Usefulness of serum lysozyme measurement in diagnosis and evaluation of sarcoidosis. N Engl J Med. 1973 Nov 15;289(20):1074–1076. doi: 10.1056/NEJM197311152892007. [DOI] [PubMed] [Google Scholar]
- Poppius H., Stenius B. Changes in arterial oxygen saturation in patients with hyperreactive airways during a histamine inhalation test. Scand J Respir Dis. 1977;58(1):1–4. [PubMed] [Google Scholar]
- Scribner D. J., Fahrney D. Neutrophil receptors for IgG and complement: their roles in the attachment and ingestion phases of phagocytosis. J Immunol. 1976 Apr;116(4):892–897. [PubMed] [Google Scholar]
- Segal A. W., Loewi G. Neutrophil dysfunction in Crohn's disease. Lancet. 1976 Jul 31;2(7979):219–221. doi: 10.1016/s0140-6736(76)91024-2. [DOI] [PubMed] [Google Scholar]
- Umbert P., Belcher R. W., Winkelmann R. K. Lymphokines (MIF) in the serum of patients with sarcoidosis and cutaneous granuloma annulare. Br J Dermatol. 1976 Nov;95(5):481–485. doi: 10.1111/j.1365-2133.1976.tb00857.x. [DOI] [PubMed] [Google Scholar]
- Venge P., Hällgren R., Stålenheim G., Olsson I. Effects of serum and cations on the selective release of granular proteins from human netrophils during phagocytosis. Scand J Haematol. 1979 Apr 4;22(4):317–326. doi: 10.1111/j.1600-0609.1979.tb00426.x. [DOI] [PubMed] [Google Scholar]


