Abstract
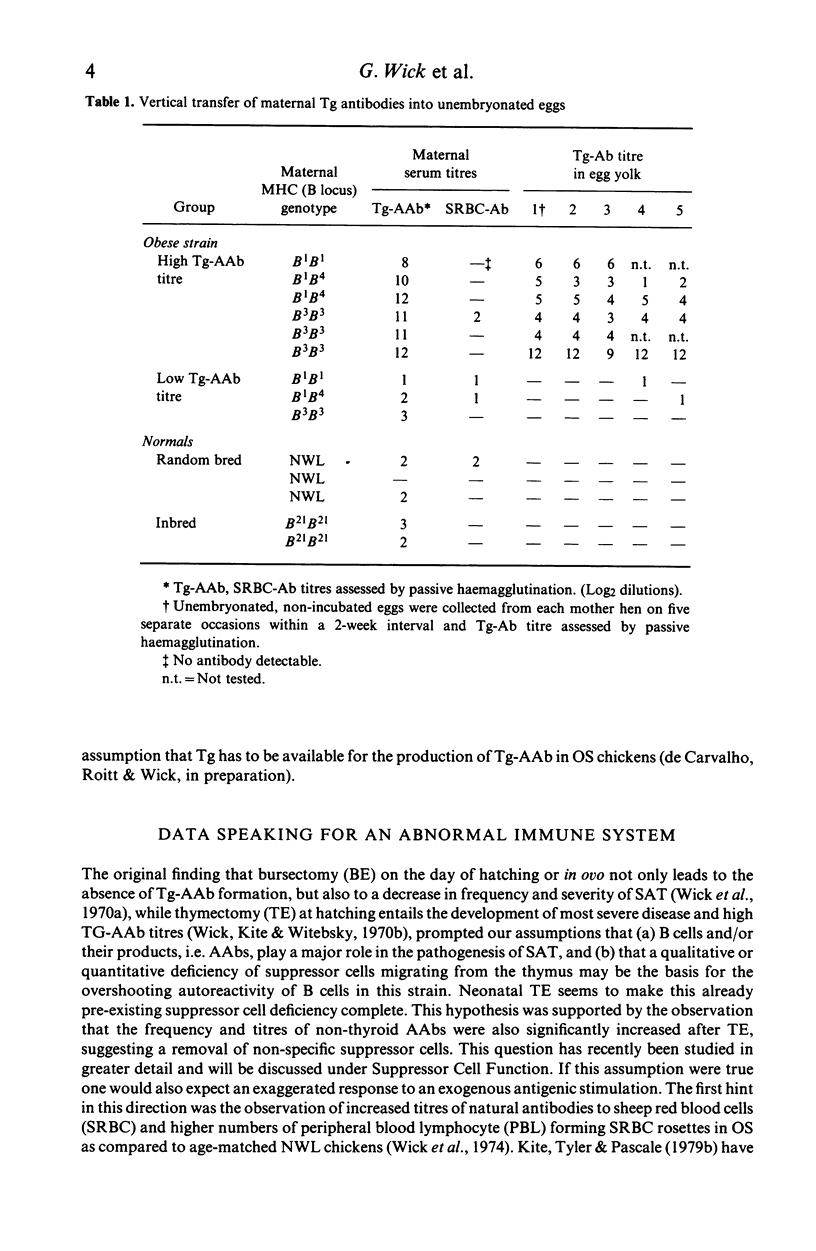
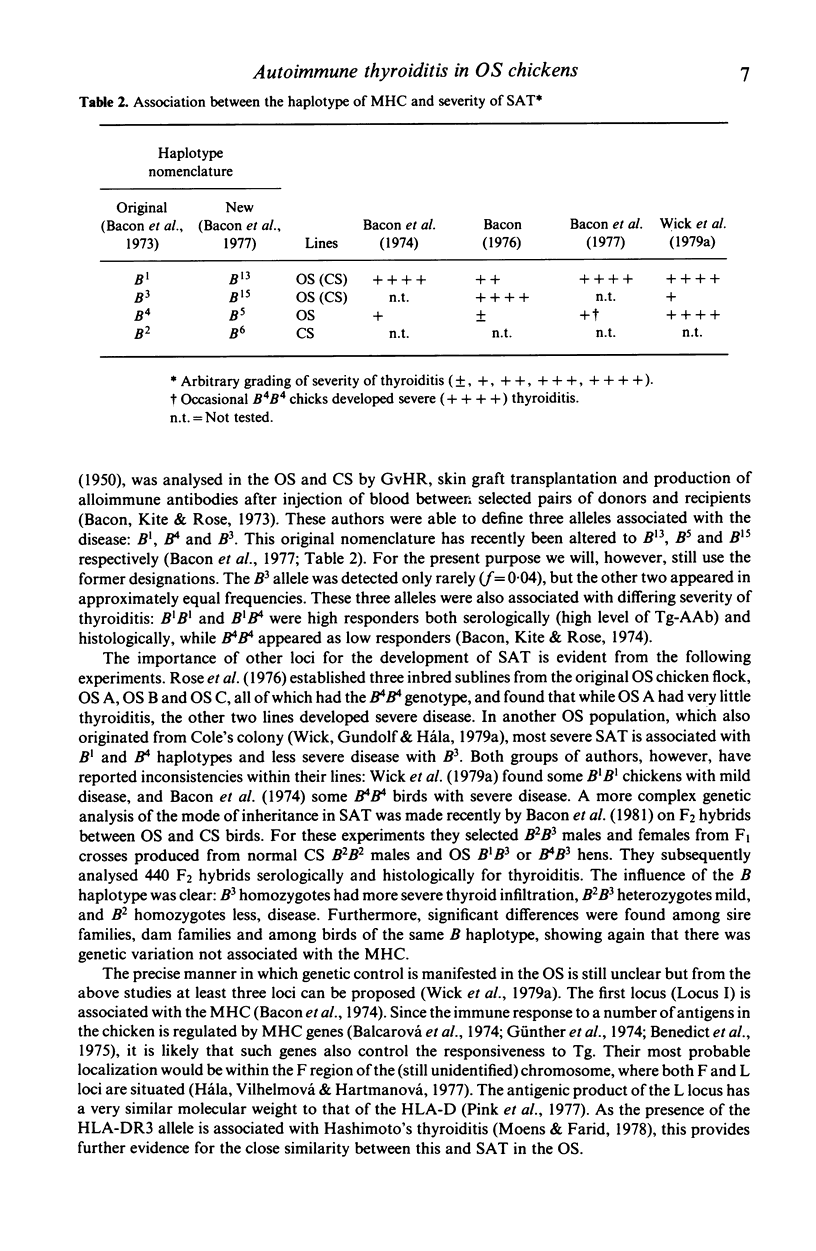
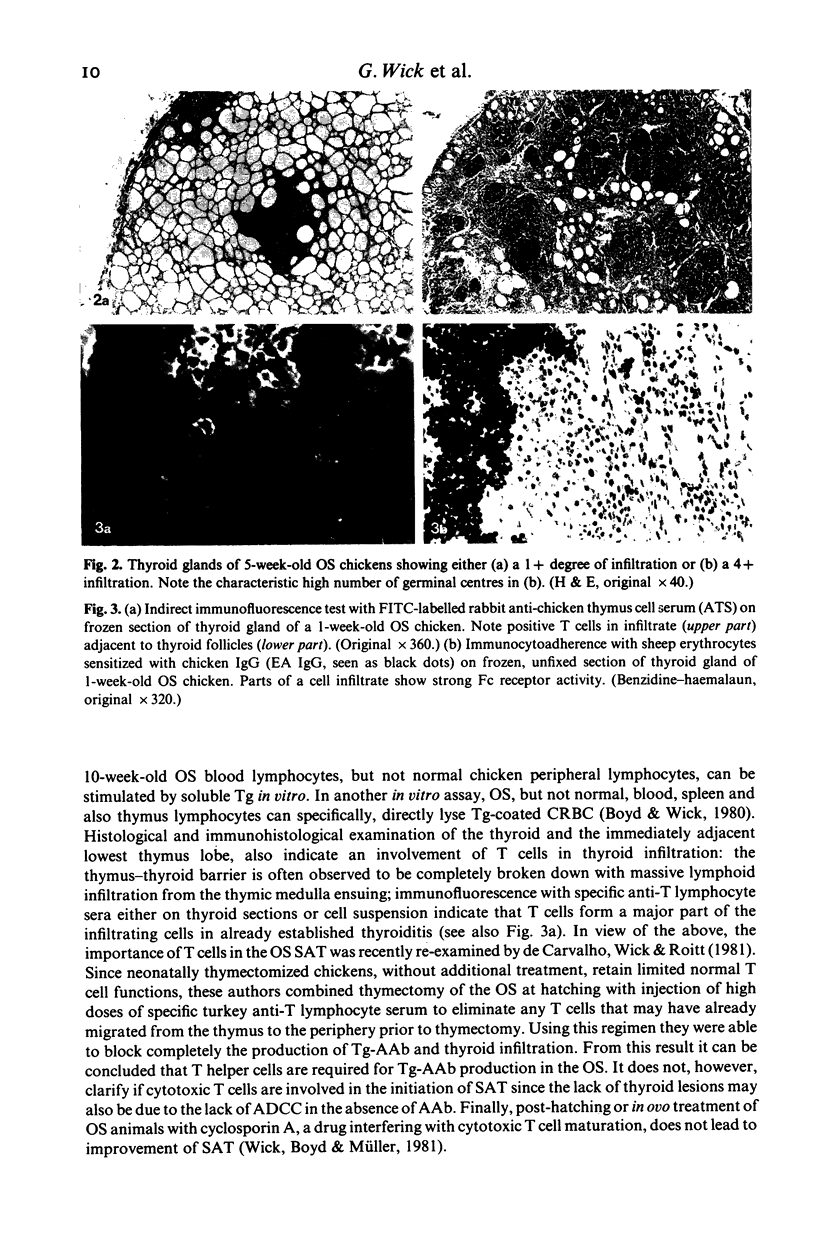
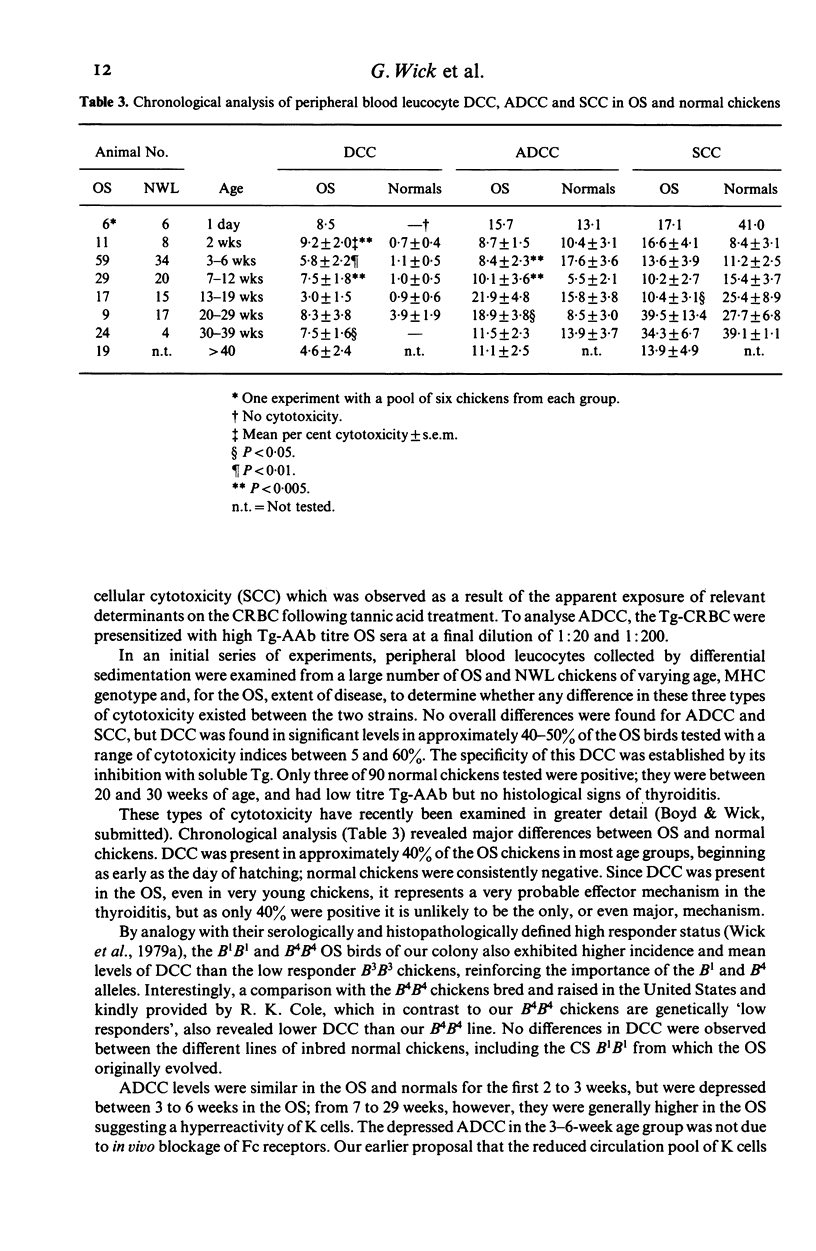
Chickens of the Obese strain (OS) develop a spontaneous, hereditary autoimmune thyroiditis during the first weeks of life which parallels human Hashimoto's thyroiditis in all clinical, histopathological and serological aspects. This review summarizes the results from investigations on this strain with special emphasis on the pathogenic effector mechanisms leading to the autoimmune destruction of the thyroid gland. The fact that this model disease arises in an avian species is particularly advantageous because of the clear-cut anatomical and functional division of the immune system. Data are discussed which suggest the following pathogenesis of spontaneous autoimmune thyroiditis: B cells and their products, i.e. thyroglobulin autoantibodies, play a decisive role in the initial phases of the disease, such antibodies are first vertically transferred from the mother hen via the egg yolk into the embryo and newly hatched chick, the immune system of which then takes over their production. T-helper cells are required for the formation of the thyroglobulin autoantibodies. These antibodies can either be complement-fixing or mediate destruction of the target cells via antibody-dependent cellular cytotoxicity (ADCC). B cells themselves also destroy thyroid epithelial cells directly in ADCC-like fashion. Cytotoxic T cells seem to play a minor role in the beginning, but add to the destruction of the thyroid gland later. The underlying defect appears to be a deficient intrathymic maturation of suppressor cells leading to a lack of their emigration to the periphery. The development of the disease is under multigenic control, involving at least three loci. Genes associated with the B locus [the major histocompatibility complex (MHC) of the chicken] and a non-B locus seem to be responsible for the immunological hyperreactivity of the OS towards autologous (thyroid and non-thyroid) and exogenous antigens. The third locus is considered to determine a primary thyroid defect thus predisposing this organ as a prime target for the manifestation of autoimmune disease in the OS.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albini B., Wick G. Proportional increase of bursa-derived cells in chickens of the Obese strain. Nature. 1974 Jun 14;249(458):653–654. doi: 10.1038/249653a0. [DOI] [PubMed] [Google Scholar]
- BRILES W. E., McGIBBON W. H., IRWIN M. R. On multiple alleles effecting cellular antigens in the chicken. Genetics. 1950 Nov;35(6):633–652. doi: 10.1093/genetics/35.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon L. D., Kite J. H., Jr, Rose N. R. Immunogenetic detection of B locus genotypes in chickens with autoimmune thyroiditis. Transplantation. 1973 Dec;16(6):591–598. doi: 10.1097/00007890-197312000-00010. [DOI] [PubMed] [Google Scholar]
- Bacon L. D., Kite J. H., Jr, Rose N. R. Relation between the major histocompatibility (B) locus and autoimmune thyroiditis in obese chickens. Science. 1974 Oct 18;186(4160):274–275. doi: 10.1126/science.186.4160.274. [DOI] [PubMed] [Google Scholar]
- Bacon L. D., Sundick R. S., Rose N. R. Genetic and cellular control of spontaneous autoimmune thyroiditis in OS chickens. Adv Exp Med Biol. 1977;88:309–318. doi: 10.1007/978-1-4613-4169-7_29. [DOI] [PubMed] [Google Scholar]
- Balcarová J., Derka J., Hála K., Hraba T. Genetic control of immune response to the dinitrophenol group inbred lines of chickens. Folia Biol (Praha) 1974;20(5):346–349. [PubMed] [Google Scholar]
- Boyd R., Wick G. Killer cells in the chicken: a microcytotoxicity assay using antigen-coated erythrocytes as targets. J Immunol Methods. 1980;35(3-4):233–247. doi: 10.1016/0022-1759(80)90250-1. [DOI] [PubMed] [Google Scholar]
- Calder B. A., Penhale W. J., Barnes E. W., Irvine W. J. Cytotoxic lymphocytes in Hashimoto thyroiditis. An in vitro assay system using 51 Cr-labelled chicken red blood cells coated with thyroglobulin. Clin Exp Immunol. 1973 May;14(1):19–23. [PMC free article] [PubMed] [Google Scholar]
- Cole R. K. Hereditary hypothyroidism in the domestic fowl. Genetics. 1966 Jun;53(6):1021–1033. doi: 10.1093/genetics/53.6.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. K., Kite J. H., Jr, Wick G., Witebsky E. Recent advances in avian endocrinology. 2. Inherited autoimmune thyroiditis in the fowl. Poult Sci. 1970 Jul;49(4):839–848. doi: 10.3382/ps.0490839. [DOI] [PubMed] [Google Scholar]
- Flanagan T. D., Barron A. L., Kite J. H., Witebsky E. Virological investigations of chickens with spontaneous autoimmune thyroiditis. I. Isolation and transmission studies. Avian Dis. 1970 Nov;14(4):613–616. [PubMed] [Google Scholar]
- Günther E., Balcarová J., Hála K., Rüde E., Hraba T. Evidence for an association between immune responsiveness of chicken to (T, G)-A--L and the major histocompatibility system. Eur J Immunol. 1974 Aug;4(8):548–553. doi: 10.1002/eji.1830040806. [DOI] [PubMed] [Google Scholar]
- Hashizume K., Fenzi G., DeGroot L. J. Thyroglobulin inhibition of thyrotropin binding to thyroid plasma membrane. J Clin Endocrinol Metab. 1978 Apr;46(4):679–685. doi: 10.1210/jcem-46-4-679. [DOI] [PubMed] [Google Scholar]
- Hála K., Vilhelmová M., Hartmanová J. The structure of the major histocompatibility complex of the chicken. Adv Exp Med Biol. 1977;88:227–232. doi: 10.1007/978-1-4613-4169-7_21. [DOI] [PubMed] [Google Scholar]
- Jakobisiak M., Sundick R. S., Bacon L. D., Rose N. R. Abnormal response to minor histocompatibility antigens in Obese strain chickens. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2877–2880. doi: 10.1073/pnas.73.8.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaroszewski J., Sundick R. S., Rose N. R. Effects of antiserum containing thyroglobulin antibody on the chicken thyroid gland. Clin Immunol Immunopathol. 1978 May;10(1):95–103. doi: 10.1016/0090-1229(78)90013-2. [DOI] [PubMed] [Google Scholar]
- Keast D., Ayre D. J. Antibody regulation in birds by thyroid hormones. Dev Comp Immunol. 1980 Spring;4(2):323–330. doi: 10.1016/s0145-305x(80)80035-8. [DOI] [PubMed] [Google Scholar]
- Kofler R., Wick G. Immunofluorescent localization of thyroglobulin--autoantibody producing cells in various organs of obese strain (OS) chickens. Z Immunitatsforsch Immunobiol. 1978;154(1):88–93. [PubMed] [Google Scholar]
- Kofler R., Wick G. Some methodological aspects of the haemolytic plaque forming cell assay in the chicken system [proceedings]. Folia Biol (Praha) 1979;25(5):337–339. [PubMed] [Google Scholar]
- Kong Y., David C. S., Giraldo A. A., Elrehewy M., Rose N. R. Regulation of autoimmune response to mouse thyroglobulin: influence of H-2D-end genes. J Immunol. 1979 Jul;123(1):15–18. [PubMed] [Google Scholar]
- Lee L. F. Chicken lymphocyte stimulation by mitogens: a microassay with whole-blood cultures. Avian Dis. 1978 Apr-Jun;22(2):296–307. [PubMed] [Google Scholar]
- Longenecker B. M., Pazderka F., Law G. R., Ruth R. F. Genetic control of graft-versus-host competence. Transplantation. 1972 Oct;14(4):424–431. doi: 10.1097/00007890-197210000-00003. [DOI] [PubMed] [Google Scholar]
- Luster M. I., Bacon L. D., Rose N. R., Leslie G. A. Immunogenetic and ontogenetic studies of chickens with selective IgA deficiency and autoimmune thyroiditis. Cell Immunol. 1977 Aug;32(2):417–423. doi: 10.1016/0008-8749(77)90217-9. [DOI] [PubMed] [Google Scholar]
- Moens H., Farid N. R., Sampson L., Noel E. P., Barnard J. M. Hashimoto's thyroiditis is associated with HLA-DRw3. N Engl J Med. 1978 Jul 20;299(3):133–134. doi: 10.1056/NEJM197807202990306. [DOI] [PubMed] [Google Scholar]
- Nakamura R. M., Weigle W. O. Transfer of experimental autoimmune thyroiditis by serum from thyroidectomized donors. J Exp Med. 1969 Aug 1;130(2):263–285. doi: 10.1084/jem.130.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak J. S., Bacon L. D., Rose N. R. Fc receptor-bearing lymphoid cells in the chicken. II. Relative increase of Fc(IgG) receptor bearing cells in obese strain-chickens. Immunol Commun. 1978;7(6):621–633. doi: 10.3109/08820137809068723. [DOI] [PubMed] [Google Scholar]
- Perlmann H., Perlmann P., Pape G. R., Halldén G. Purification, fractionation and assay of antibody-dependent lymphocytic effector cells (K cells) in human blood. Scand J Immunol. 1976 Jun;Suppl 5:57–68. doi: 10.1111/j.1365-3083.1976.tb03856.x. [DOI] [PubMed] [Google Scholar]
- Richter E., Wick G., Schauenstein K. The nature of active and passive thyroglobulin binding lymphoid cells in Obese strain (OS) chickens. Eur J Immunol. 1975 Aug;5(8):554–559. doi: 10.1002/eji.1830050810. [DOI] [PubMed] [Google Scholar]
- Richter E., Wick G. Thyroglobulin-binding lymphoid cells in obese strain (OS) chickens. J Immunol. 1975 Feb;114(2 Pt 2):757–761. [PubMed] [Google Scholar]
- Rose N. R., Bacon L. D., Sundick R. S. Genetic determinants of thyroiditis in the OS chicken. Transplant Rev. 1976;31:264–285. doi: 10.1111/j.1600-065x.1976.tb01457.x. [DOI] [PubMed] [Google Scholar]
- Scanes C. G., Gales L., Harvey S., Chadwick A., Newcomer W. S. Endocrine studies in young chickens of the obese strain. Gen Comp Endocrinol. 1976 Dec;30(4):419–423. doi: 10.1016/0016-6480(76)90110-6. [DOI] [PubMed] [Google Scholar]
- Schauenstein K., Wick G. Local production of immunoglobulin in the thyroid gland of obese strain (OS) chickens. Clin Exp Immunol. 1974 Aug;17(4):637–646. [PMC free article] [PubMed] [Google Scholar]
- Snell G. D. The H-2 locus of the mouse: observations and speculations concerning its comparative genetics and its polymorphism. Folia Biol (Praha) 1968;14(5):335–358. [PubMed] [Google Scholar]
- Sundick R. S., Bagchi N., Livezey M. D., Brown T. R., Mack R. E. Abnormal thyroid regulation in chickens with autoimmune thyroiditis. Endocrinology. 1979 Aug;105(2):493–498. doi: 10.1210/endo-105-2-493. [DOI] [PubMed] [Google Scholar]
- Sundick R. S., Wick G. Increased 131I uptake by the thyroid glands of Obese strain (OS) chickens derived from non-Protamone-supplemented hens. Clin Exp Immunol. 1974 Sep;18(1):127–139. [PMC free article] [PubMed] [Google Scholar]
- Sundick R. S., Wick G. Increased iodine uptake by obese strain thyroid glands transplanted to normal chick embryos. J Immunol. 1976 May;116(5):1319–1323. [PubMed] [Google Scholar]
- Tomazic V., Rose N. R. Autoimmune murine thyroiditis. VIII. Role of different thyroid antigens in the induction of experimental autoimmune thyroiditis. Immunology. 1976 Jan;30(1):63–68. [PMC free article] [PubMed] [Google Scholar]
- Tomazic V., Rose N. R., Shreffler D. C. Autoimmune murine thyroiditis. IV. Localization of genetic control of the immune response. J Immunol. 1974 Mar;112(3):965–969. [PubMed] [Google Scholar]
- VAN TIENHOVEN A., COLE R. K. Endocrine disturbances in obese chickens. Anat Rec. 1962 Feb;142:111–121. doi: 10.1002/ar.1091420203. [DOI] [PubMed] [Google Scholar]
- Wick G., Graf J. Electron microscopic studies in chickens of the obese strain with spontaneous hereditary autoimmune thyroiditis. Lab Invest. 1972 Oct;27(4):400–411. [PubMed] [Google Scholar]
- Wick G., Gundolf R., Hála K. Genetic factors in spontaneous autoimmune thyroiditis in OS chickens. J Immunogenet. 1979 Jun;6(3):177–183. doi: 10.1111/j.1744-313x.1979.tb00343.x. [DOI] [PubMed] [Google Scholar]
- Wick G., Kite J. H., Jr, Witebsky E. Spontaneous thyroiditis in the obese strain of chickens. IV. The effect of thymectomy and thymo-bursectomy on the development of the disease. J Immunol. 1970 Jan;104(1):54–62. [PubMed] [Google Scholar]
- Wick G., Sundick R. S., Albini B. A review: The obese strain (OS) of chickens: an animal model with spontaneous autoimmune thyroiditis. Clin Immunol Immunopathol. 1974 Nov;3(2):272–300. doi: 10.1016/0090-1229(74)90015-4. [DOI] [PubMed] [Google Scholar]
- Witebsky E., Kite J. H., Jr, Wick G., Cole R. K. Spontaneous thyroiditis in the obese strain of chichens. I. Demonstration of circulating autoantibodies. J Immunol. 1969 Oct;103(4):708–715. [PubMed] [Google Scholar]
- Witebsky E. The clinical pathology of autoimmunization. Ward Burdick award address. Am J Clin Pathol. 1968 Mar;49(3):301–311. doi: 10.1093/ajcp/49.3.301. [DOI] [PubMed] [Google Scholar]
- Zeigel R. F., Barron A. L., Kite J. H., Witebsky E. Virological investigations of chickens with spontaneous autoimmune thyroiditis. II. Examination of tissues by electron microscopy. Avian Dis. 1970 Nov;14(4):617–619. [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]
- de Carvalho L. C., Wick G., Roitt I. M. Requirement of T cells for the development of spontaneous autoimmune thyroiditis in obese strain (OS) chickens. J Immunol. 1981 Feb;126(2):750–753. [PubMed] [Google Scholar]





