Abstract
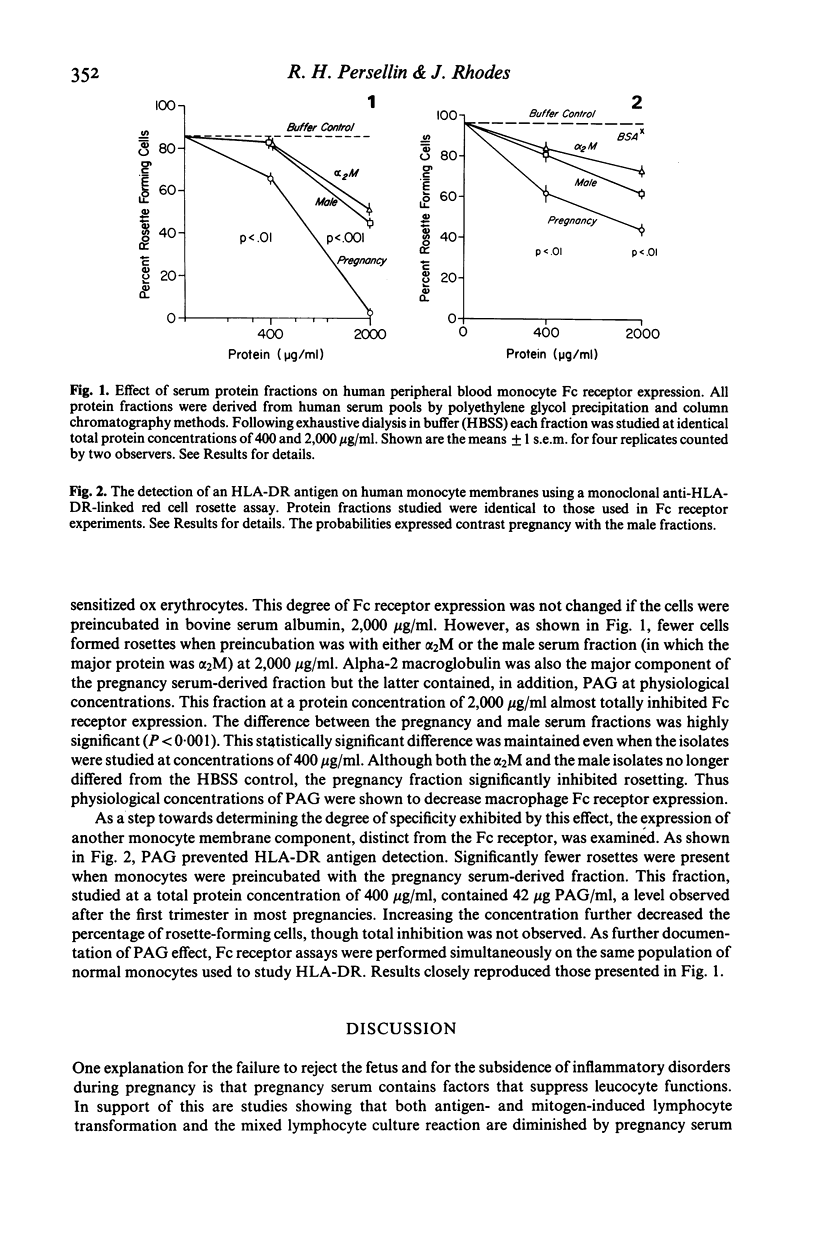
The precise factor responsible for the altered immunological reactivity and diminished inflammatory responses occurring in pregnancy is unknown. Recent observations implicate the pregnancy-associated alpha-2 glycoprotein (PAG). Using rosette assays for the detection of cells carrying Fc receptors and for the demonstration of surface HLA-DR antigens, we demonstrate that a serum protein fraction rich in PAG inhibits these surface markers on human monocytes. Brief exposure of normal peripheral blood monocytes to physiological concentrations of PAG led to almost total loss of Fc receptor expression, an effect that was concentration-related. Using a monoclonal anti-HLA-DR antibody linked to red cells, physiological concentrations of PAG also significantly inhibited the detection of HLA-DR on monocytes. As controls, we used a protein fraction isolated in identical fashion from male sera devoid of PAG, and an alpha-2 macroglobulin isolate. These findings suggest that PAG, by masking monocyte surface markers, could be responsible for the suppression of some cell functions during pregnancy.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beer A. E., Billingham R. E. Immunoregulatory aspects of pregnancy. Fed Proc. 1978 Aug;37(10):2374–2378. [PubMed] [Google Scholar]
- Björksten B., Söderström T., Damber M. G., von Schoultz B., Stigbrand T. Polymorphonuclear leucocyte function during pregnancy. Scand J Immunol. 1978;8(3):257–262. doi: 10.1111/j.1365-3083.1978.tb00518.x. [DOI] [PubMed] [Google Scholar]
- Coombs R. R., Wilson A. B., Eremin O., Gurner B. W., Haegert D. G., Lawson Y. A., Bright S., Munro A. J. Comparison of the direct antiglobulin rosetting reaction with the mixed antiglobulin rosetting reaction for the detection of immunoglobulin on lymphocytes. J Immunol Methods. 1977;18(1-2):45–54. doi: 10.1016/0022-1759(77)90157-0. [DOI] [PubMed] [Google Scholar]
- Fujisaki S., Mori N., Sasaki T., Maeyama M. Cell-mediated immunity in human pregnancy: Changes in lymphocyte reactivity during pregnancy and postpartum. Microbiol Immunol. 1979;23(9):899–907. doi: 10.1111/j.1348-0421.1979.tb02823.x. [DOI] [PubMed] [Google Scholar]
- Kasakura S. A factor in maternal plasma during pregnancy that suppresses the reactivity of mixed leukocyte cultures. J Immunol. 1971 Nov;107(5):1296–1301. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Neely N. T., Persellin R. H. Activity of rheumatoid arthritis during pregnancy. Tex Med. 1977 Aug;73(8):59–63. [PubMed] [Google Scholar]
- Persellin R. H., Leibfarth J. K. Studies of the effects of pregnancy serum on polymorphonuclear leukocyte functions. Arthritis Rheum. 1978 Apr;21(3):316–325. doi: 10.1002/art.1780210305. [DOI] [PubMed] [Google Scholar]
- Persellin R. H. The effect of pregnancy on rheumatoid arthritis. Bull Rheum Dis. 1976;27(9):922–927. [PubMed] [Google Scholar]
- Persellin R. H., Thorne K. J. Inhibition of phagocytosis of Trypanosome dionisii by pregnancy alpha-2 glycoprotein. Trans R Soc Trop Med Hyg. 1981;75(3):436–438. doi: 10.1016/0035-9203(81)90114-0. [DOI] [PubMed] [Google Scholar]
- Purtilo D. T., Hallgren H. M., Yunis E. J. Depressed maternal lymphocyte response to phytohaemagglutinin in human pregnancy. Lancet. 1972 Apr 8;1(7754):769–771. doi: 10.1016/s0140-6736(72)90522-3. [DOI] [PubMed] [Google Scholar]
- Rhodes J. Altered expression of human monocyte Fc receptors in malignant disease. Nature. 1977 Jan 20;265(5591):253–255. doi: 10.1038/265253a0. [DOI] [PubMed] [Google Scholar]
- Rhodes J., Bishop M., Benfield J. Tumor surveillance: how tumors may resist macrophage-mediated host defense. Science. 1979 Jan 12;203(4376):179–182. doi: 10.1126/science.758686. [DOI] [PubMed] [Google Scholar]
- Stimson W. H. Identification of pregnancy-associated alpha-macroglobulin on the surface of peripheral blood leucocyte populations. Clin Exp Immunol. 1977 Jun;28(3):445–452. [PMC free article] [PubMed] [Google Scholar]
- Stimson W. H. Studies on the immunosuppressive properties of a pregnancy-associated alpha-macroglobulin. Clin Exp Immunol. 1976 Aug;25(2):199–206. [PMC free article] [PubMed] [Google Scholar]
- Stites D. P., Pavia C. S., Clemens L. E., Kuhn R. W., Siiteri P. K. Immunologic regulation in pregnancy. Arthritis Rheum. 1979 Nov;22(11):1300–1307. doi: 10.1002/art.1780221119. [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Persellin R. H. The inhibitory effect of pregnancy serum on polymorphonuclear leukocyte chemotaxis. J Clin Lab Immunol. 1980 Mar;3(2):121–124. [PubMed] [Google Scholar]
- Than G. N., Csaba I. F., Karg N. J., Szabo D. G., Ambrus M., Bajtai G. Letter: Immunosuppressive effect of pregnancy-associated alpha-glycoprotein. Lancet. 1975 Sep 13;2(7933):515–515. doi: 10.1016/s0140-6736(75)90606-6. [DOI] [PubMed] [Google Scholar]
- Thomson A. W., Hunter C. B., Cruickshank N., Horne C. H. Study of pregnancy-associated alpha2-glycoprotein in relation to populations of human blood leucocytes. Int Arch Allergy Appl Immunol. 1979;58(3):251–259. doi: 10.1159/000232201. [DOI] [PubMed] [Google Scholar]
- Von Schoultz B., Stigbrand T., Tärnvik A. Inhibition of PHA-induced lymphocyte stimulation by the pregnancy zone protein. FEBS Lett. 1973 Dec 15;38(1):23–26. doi: 10.1016/0014-5793(73)80503-4. [DOI] [PubMed] [Google Scholar]


