Abstract
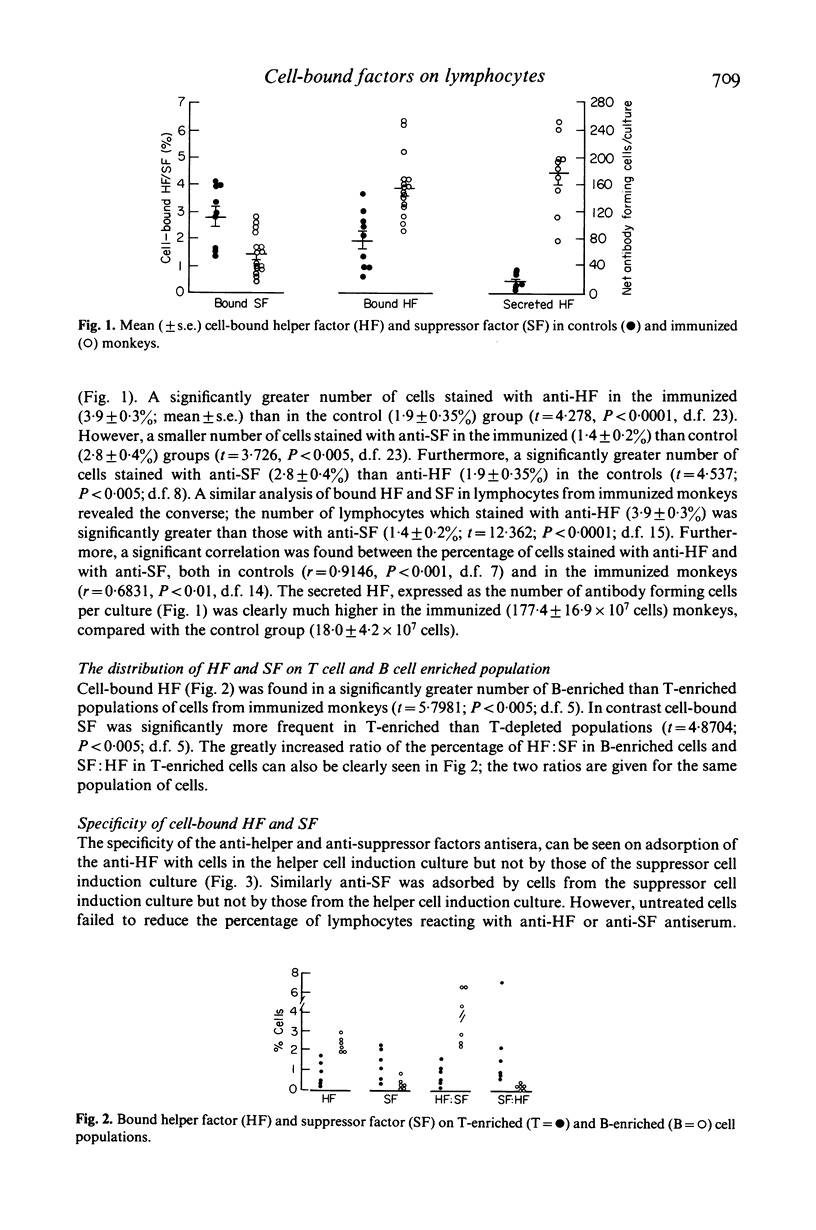
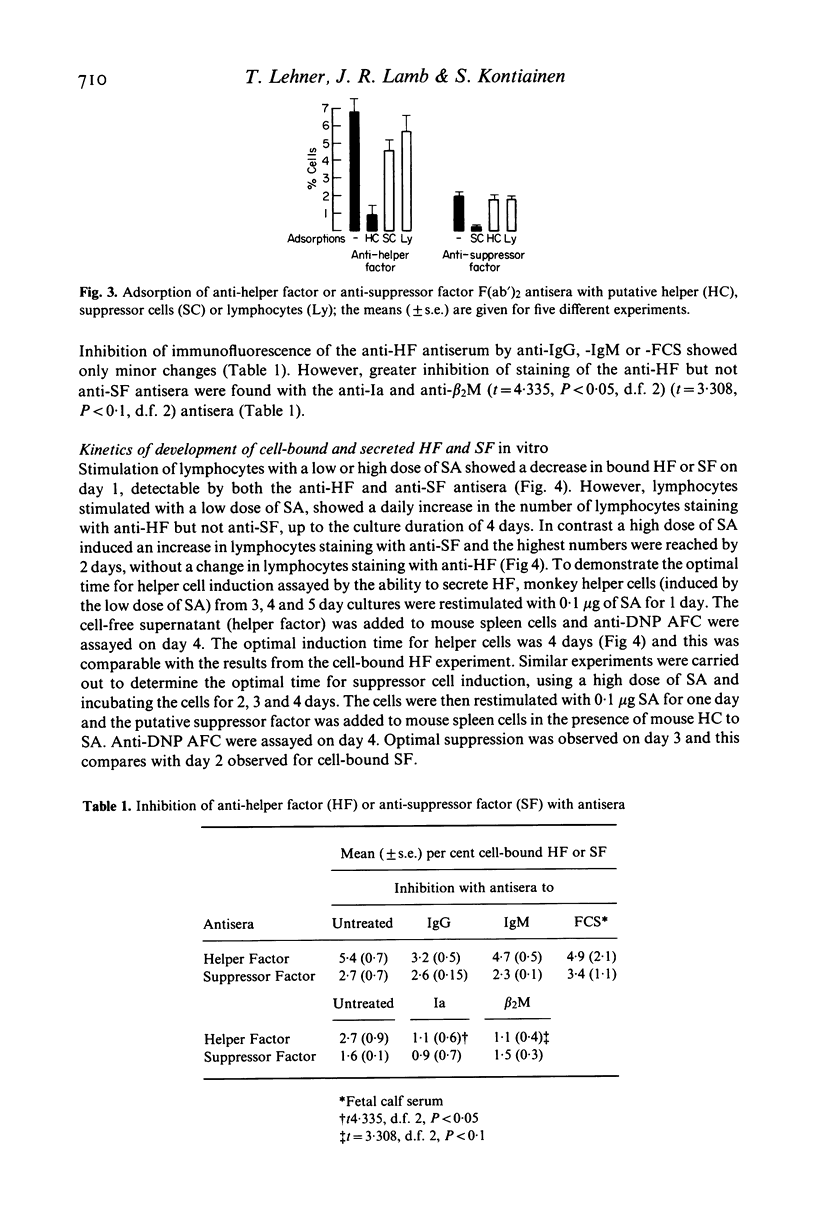
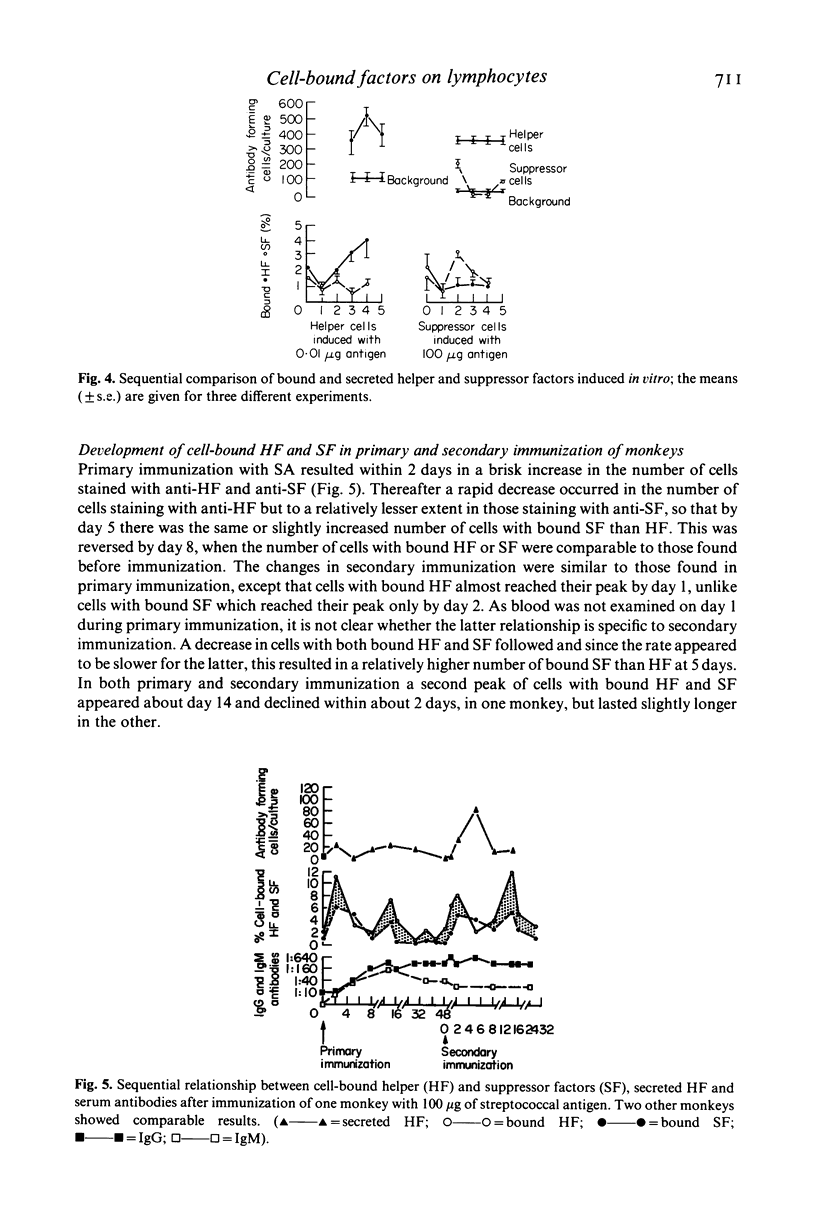
Cell-bound helper and suppressor factors, on lymphocytes from Rhesus monkeys, were assayed by the indirect immunofluorescent method, using F(ab')2 fragments of rabbit antisera to antigen specific secreted helper and suppressor factors. The rabbit antisera recognize the function related to the 'constant' region of secreted antigen specific helper or suppressor factor. Immunoadsorption studies suggest that the two antisera also recognize cell surface markers of helper or suppressor cell, for the activity of the anti-helper factor (HF) antiserum was adsorbed by cells from helper cell but not by suppressor cell induction cultures and the converse was found for the anti-suppressor factor (SF) antiserum. A significantly greater proportion of bound SF than HF was found in lymphocytes from controls and the converse or a greater proportion of HF and SF was found in lymphocytes from immunized monkeys. Furthermore, a significantly greater proportion of T-enriched cells bound anti-SF than anti-HF and the converse was found with T-depleted cells, suggesting that the assay detects the target cell as well as the cell of origin of these factors. A sequential in vitro comparison of cell-bound and secreted factors revealed that the highest number of cells which stained with anti-HF was on day 4 of the culture and this correlated with the highest secreted HF activity. However, cells which stained with anti-SF reached a peak on day 2, whereas maximal secreted SF activity was found on day 3. In vivo the kinetic relationships after immunization showed similar timings for the development of bound HF and SF, though the proportion of cells differed. However, the secreted HF after secondary immunization reached its highest level of activity on day 5, as compared with day 2 for cell bound HF. Both primary and secondary cellular but not antibody responses showed well defined bi-phasic responses and their significance as well as those of bound HF and SF in immunoregulation will need to be explored further.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Britton S., Möller G. Regulation of antibody synthesis against Escherichia coli endotoxin. I. Suppressive effect of endogenously produced and passively transferred antibodies. J Immunol. 1968 Jun;100(6):1326–1334. [PubMed] [Google Scholar]
- Britton S., Wepsic T., Möller G. Persistence of immunogenicity of two complex antigens retained in vivo. Immunology. 1968 Apr;14(4):491–501. [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Boyse E. Regulation of the immune response by T-cell subclasses. Contemp Top Immunobiol. 1977;7:47–67. doi: 10.1007/978-1-4684-3054-7_2. [DOI] [PubMed] [Google Scholar]
- Feldmann M., Beverley P., Erb P., Howie S., Kontiainen S., Maoz A., Mathies M., McKenzie I., Woody J. Current concepts of the antibody response: heterogeneity of lymphoid cells, interactions, and factors. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):113–118. doi: 10.1101/sqb.1977.041.01.015. [DOI] [PubMed] [Google Scholar]
- Howie S., Feldmann M. Immune response (Ir) genes expressed at macrophage-B lymphocyte interactions. Nature. 1978 Jun 22;273(5664):664–666. doi: 10.1038/273664a0. [DOI] [PubMed] [Google Scholar]
- Howie S., Parish C. R., David C. S., McKenzie I. F., Maurer P. H., Feldmann M. Serological analysis of antigen-specific helper factors specific for poly-L(Tyr, Glu)-poly-DLAla--poly-LLys [(T, G)-A--L] and L Glu60-LAla30-LTyr10 (GAT). Eur J Immunol. 1979 Jul;9(7):501–506. doi: 10.1002/eji.1830090703. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kantor F., Feldmann M. Induction of human antigen-specific and non-specific helper factors in vitro. Clin Exp Immunol. 1979 Apr;36(1):71–77. [PMC free article] [PubMed] [Google Scholar]
- Kontiainen S., Feldmann M. Structural characteristics of antigen-specific suppressor factors: definition of 'constant' region and 'variable' region determinants. Thymus. 1979 Sep;1(1-2):59–79. [PubMed] [Google Scholar]
- Kontiainen S., Feldmann M. Suppressor cell induction in vitro. I. Kinetics of induction of antigen-specific suppressor cells. Eur J Immunol. 1976 Apr;6(4):296–301. doi: 10.1002/eji.1830060412. [DOI] [PubMed] [Google Scholar]
- Kontiainen S., Woody J. N., Rees A., Feldmann M. Induction of human antigen-specific suppressor factors in vitro. Clin Exp Immunol. 1981 Mar;43(3):517–525. [PMC free article] [PubMed] [Google Scholar]
- Lamb J. R., Kontiainen S., Lehner T. A comparative investigation of the generation of specific T cell-helper function induced by Streptococcus mutans in monkeys and mice. J Immunol. 1980 May;124(5):2384–2389. [PubMed] [Google Scholar]
- Lamb J. R., Kontiainen S., Lehner T. Generation of specfic T-cell suppressor function induced by Streptococcus mutans in monkeys and mice. Infect Immun. 1979 Dec;26(3):903–909. doi: 10.1128/iai.26.3.903-909.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. R., Zanders E. D., Sanderson A. R., Ward P. J., Feldmann M., Kontiainen S., Lehner T., Woody J. N. Antigen-specific helper factor reacts with antibodies to human beta 2 microglobulin. J Immunol. 1981 Jul;127(1):231–234. [PubMed] [Google Scholar]
- Lehner T., Challacombe S. J., Wilton J. M., Caldwell J. Cellular and humoral immune responses in vaccination against dental caries in monkeys. Nature. 1976 Nov 4;264(5581):69–72. doi: 10.1038/264069a0. [DOI] [PubMed] [Google Scholar]
- Lehner T., Lamb J. R., Welsh K. L., Batchelor R. J. Association between HLA-DR antigens and helper cell activity in the control of dental caries. Nature. 1981 Aug 20;292(5825):770–772. doi: 10.1038/292770a0. [DOI] [PubMed] [Google Scholar]
- Mudawwar F. B., Yunis E. J., Geha R. S. Antigen-specific helper factor in man. J Exp Med. 1978 Oct 1;148(4):1032–1043. doi: 10.1084/jem.148.4.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro A. J., Taussig M. J. Two genes in the major histocompatibility complex control immune response. Nature. 1975 Jul 10;256(5513):103–106. doi: 10.1038/256103a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M. W., Lehner T. Characterisation of antigens extracted from cells and culture fluids of Streptococcus mutans serotype c. Arch Oral Biol. 1978;23(1):7–15. doi: 10.1016/0003-9969(78)90047-x. [DOI] [PubMed] [Google Scholar]
- Wilson B. J., Kocvara H. A simple rapid method for layering blood on Ficoll-Isopaque gradients. J Immunol Methods. 1975 Nov;9(1):67–68. doi: 10.1016/0022-1759(75)90036-8. [DOI] [PubMed] [Google Scholar]
- Zanders E. D., Lamb J. R., Kontiainen S., Lehner T. Partial characterization of murine and monkey helper factor to a streptococcal antigen. Immunology. 1980 Nov;41(3):587–596. [PMC free article] [PubMed] [Google Scholar]


