Abstract
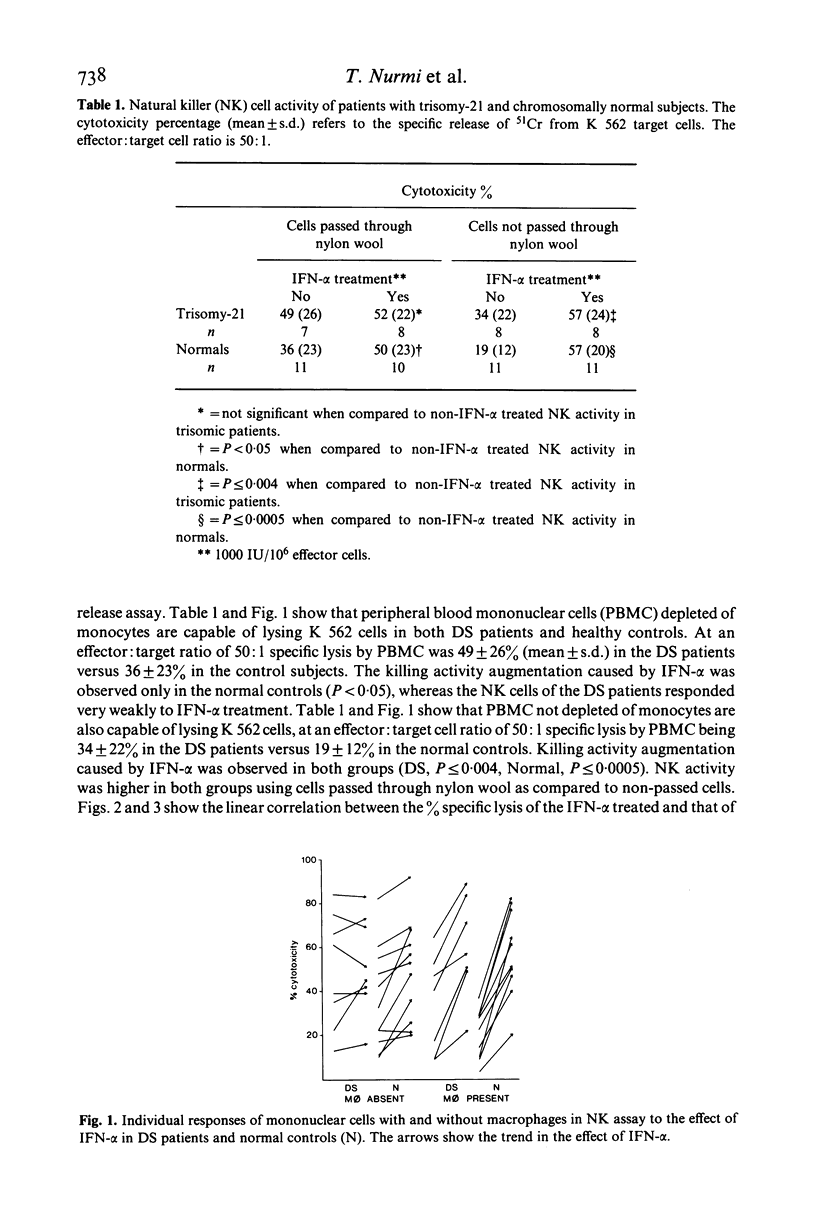
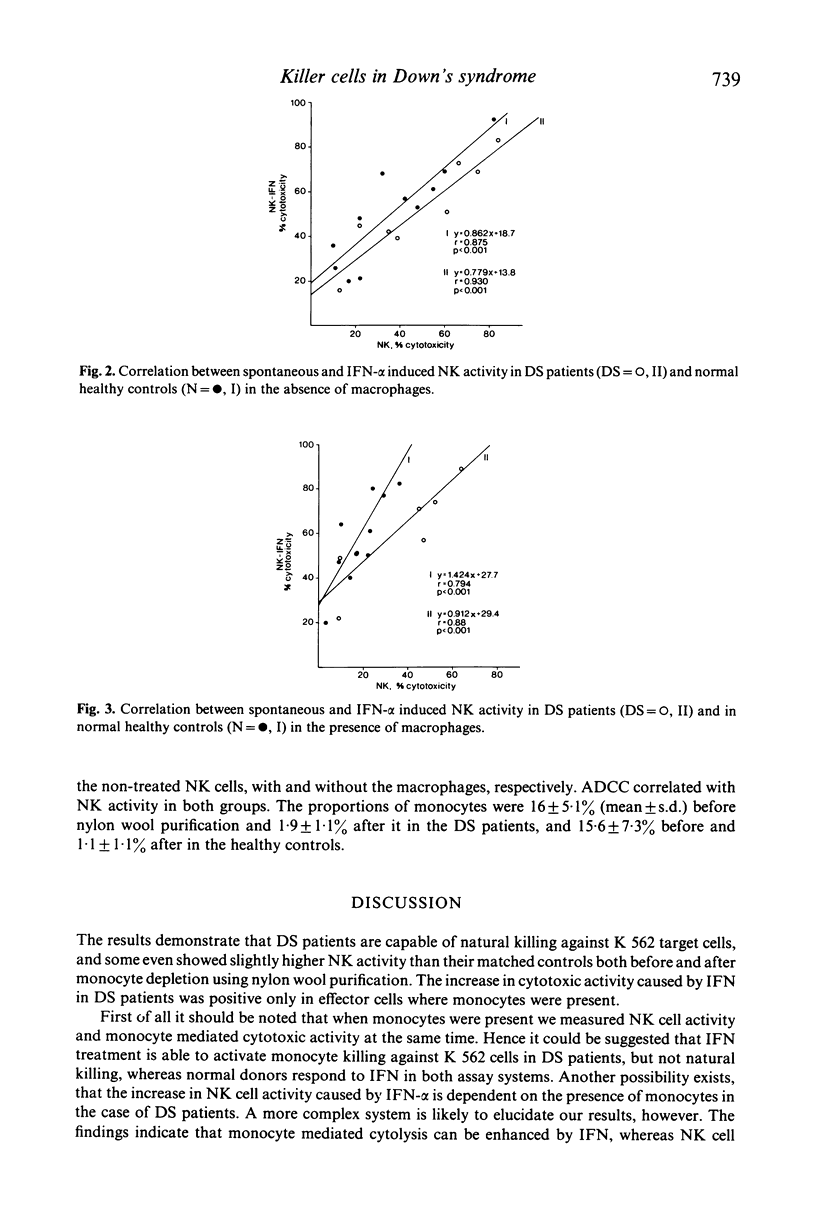
Natural killer (NK) activity and antibody-dependent cell mediated cytotoxicity (ADCC) against a human myeloid target cell line (K 562) was measured in adult patients with trisomy-21 (Down's syndrome) and in chromosomally normal age and sex matched control subjects. The effect of human leucocyte interferon (IFN-alpha) on the NK activity was also estimated. Spontaneous NK activity was stronger in the adult patients with trisomy-21 than in the healthy controls, but the difference did not reach statistical significance. The augmentation of NK activity by IFN-alpha, measured using lymphocytes not depleted of monocytes as effector cells, was statistically significant in both the trisomic patients (P less than 0.004) and the healthy controls (P less than 0.0005). Using monocyte and macrophage depleted lymphocytes in the patients with trisomy-21 the NK activity proved stronger than in the healthy controls, but not significantly and IFN-alpha did not augment it as it did in the healthy controls (P = n.s., P less than 0.05), for augmentations respectively). These results support the view that monocytes and macrophages are connected with the NK cell system. ADCC correlated with NK activity in both groups. Since NK cells are important components of many immune processes, including tumour and virus and/or bacteria-infected cell elimination, and have regulatory functions in immune reactions, the deficient augmentation of trisomic NK cells shown in vitro with extrinsic human leucocyte interferon may, paradoxically be an explanation for the greater susceptibility of trisomic individuals to lymphatic leukaemia and virus and bacterial infections. In vivo, this could be explained by the more potent secondary suppression by the 'immune' interferon produced by the virus, bacteria and malignant cells. In other words, the potential of the 'fighting couple' of the immune system, NK cell/interferon, is perhaps disturbed genetically due to the chromosome 21.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barkin R. M., Weston W. L., Humbert J. R., Maire F. Phagocytic function in Down syndrome--I. Chemotaxis. J Ment Defic Res. 1980 Dec;24(Pt 4):243–249. doi: 10.1111/j.1365-2788.1980.tb00078.x. [DOI] [PubMed] [Google Scholar]
- Burgio G. R., Lanzavecchia A., Maccario R., Vitiello A., Plebani A., Ugazio A. G. Immunodeficiency in Down's syndrome: T-lymphocyte subset imbalance in trisomic children. Clin Exp Immunol. 1978 Aug;33(2):298–301. [PMC free article] [PubMed] [Google Scholar]
- Burgio G. R., Ugazio A. G., Nespoli L., Marcioni A. F., Bottelli A. M., Pasquali F. Derangements of immunoglobulin levels, phytohemagglutinin responsiveness and T and B cell markers in Down's syndrome at different ages. Eur J Immunol. 1975 Sep;5(9):600–603. doi: 10.1002/eji.1830050904. [DOI] [PubMed] [Google Scholar]
- Clark E. A., Sturge J. C. Phylogeny of NK cell reactivity against human and nonhuman primate lymphoblastoid cell lines: evolving and conserved target antigens. J Immunol. 1981 Mar;126(3):969–974. [PubMed] [Google Scholar]
- Duse M., Brugo M. A., Martini A., Tassi C., Ferrario C., Ugazio A. G. Immunodeficiency in Down's syndrome: low levels of serum thymic factor in trisomic children. Thymus. 1980 Dec;2(3):127–131. [PubMed] [Google Scholar]
- Epstein L. B., Epstein C. J. T-lymphocyte function and sensitivity to interferon in trisomy 21. Cell Immunol. 1980 May;51(2):303–318. doi: 10.1016/0008-8749(80)90262-2. [DOI] [PubMed] [Google Scholar]
- Epstein L. B., Lee S. H., Epstein C. J. Enhanced sensitivity of trisomy 21 monocytes to the maturation-inhibiting effect ot interferon. Cell Immunol. 1980 Mar 1;50(1):191–194. doi: 10.1016/0008-8749(80)90017-9. [DOI] [PubMed] [Google Scholar]
- Gershwin M. E., Crinella F. M., Castles J. J., Trent J. K. Immunologic characteristics of Down's syndrome. J Ment Defic Res. 1977 Dec;21(4):237–249. doi: 10.1111/j.1365-2788.1977.tb01587.x. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Pross H., Wigzell H. "Natural" killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975 Feb;5(2):117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- Levin S., Schlesinger M., Handzel Z., Hahn T., Altman Y., Czernobilsky B., Boss J. Thymic deficiency in Down's syndrome. Pediatrics. 1979 Jan;63(1):80–87. [PubMed] [Google Scholar]
- Lindahl-Magnusson P., Leary P., Gresser I. Interferon inhibits DNA synthesis induced in mouse lymphocyte suspensions by phytohaemagglutinin or by allogeneic cells. Nat New Biol. 1972 May 24;237(73):120–121. doi: 10.1038/newbio237120a0. [DOI] [PubMed] [Google Scholar]
- Oster J., Mikkelsen M., Nielsen A. Mortality and life-table in Down's syndrome. Acta Paediatr Scand. 1975 Mar;64(2):322–326. doi: 10.1111/j.1651-2227.1975.tb03842.x. [DOI] [PubMed] [Google Scholar]
- Ranki A., Tötterman T. H., Häyry P. Identification of resting human T and B lymphocytes by acid alpha-naphthyl acetate esterase staining combined with rosette formation with Staphylococcus aureus strain Cowan 1. Scand J Immunol. 1976;5(10):1129–1138. doi: 10.1111/j.1365-3083.1976.tb00254.x. [DOI] [PubMed] [Google Scholar]
- Reiser K., Whitcomb C., Robinson K., MacKenzie M. R. T and B lymphocytes in patients with Down's syndrome. Am J Ment Defic. 1976 May;80(6):613–619. [PubMed] [Google Scholar]
- Richardson P. M., McGuinness U. M., Aguayo A. J. Axons from CNS neurons regenerate into PNS grafts. Nature. 1980 Mar 20;284(5753):264–265. doi: 10.1038/284264a0. [DOI] [PubMed] [Google Scholar]
- Saksela E., Timonen T., Cantell K. Human natural killer cell activity is augmented by interferon via recruitment of 'pre-NK' cells. Scand J Immunol. 1979;10(3):257–266. doi: 10.1111/j.1365-3083.1979.tb01348.x. [DOI] [PubMed] [Google Scholar]
- Seger R., Buchinger G., Ströder J. On the influence of age on immunity in Down's syndrome. Eur J Pediatr. 1977 Jan 26;124(2):77–87. doi: 10.1007/BF00477543. [DOI] [PubMed] [Google Scholar]
- Sonnenfeld G., Mandel A. D., Merigan T. C. The immunosuppressive effect of type II mouse interferon preparations on antibody production. Cell Immunol. 1977 Dec;34(2):193–206. doi: 10.1016/0008-8749(77)90243-x. [DOI] [PubMed] [Google Scholar]
- Stiehm E. R., Fudenberg H. H. Serum levels of immune globulins in health and disease: a survey. Pediatrics. 1966 May;37(5):715–727. [PubMed] [Google Scholar]
- Talmadge J. E., Meyers K. M., Prieur D. J., Starkey J. R. Role of NK cells in tumour growth and metastasis in beige mice. Nature. 1980 Apr 17;284(5757):622–624. doi: 10.1038/284622a0. [DOI] [PubMed] [Google Scholar]
- Tan Y. H., Tischfield J., Ruddle F. H. The linkage of genes for the human interferon-induced antiviral protein and indophenol oxidase-B traits to chromosome G-21. J Exp Med. 1973 Feb 1;137(2):317–330. doi: 10.1084/jem.137.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittingham S., Pitt D. B., Sharma D. L., Mackay I. R. Stress deficiency of the T-lymphocyte system exemplified by Down syndrome. Lancet. 1977 Jan 22;1(8004):163–166. doi: 10.1016/s0140-6736(77)91763-9. [DOI] [PubMed] [Google Scholar]


