Abstract
The induction of both synaptic plasticity and memory is thought to depend on the balance between opposing molecular regulatory factors, such as protein kinases and phosphatases. Here we show that inhibition of protein phosphatase 2B (calcineurin, CaN) facilitates the induction of intermediate-term memory (ITM) and long-term memory (LTM) for tail shock-induced sensitization in Aplysia without any effect on short-term memory. To identify the molecular cascade underlying the improvement of memory by inhibition of CaN, we examined the role of extracellular signal-regulated kinase 1/2/mitogen-activated protein kinase (MAPK). Molecular experiments revealed that one pulse of serotonin, which by itself does not activate MAPK, leads to significant MAPK activation in the sensory neurons of the pleural ganglia when CaN is inhibited. Extending these observations, behavioral experiments showed that the facilitated induction of ITM and LTM produced by CaN inhibition depends on MAPK activity. These results demonstrate: (i) that CaN acts as an inhibitory constraint in the formation of long-lasting phases of memory, and (ii) that facilitated induction of ITM and LTM by CaN inhibition requires MAPK activity.
Keywords: memory enhancement‖phosphatase‖extracellular signal-regulated kinase
The induction of synaptic plasticity underlying memory formation is thought to depend on counteracting molecular regulatory steps (1). This bidirectional control in neurons operates at least at two levels: transcriptional regulation by activator and repressor forms of transcription factors (2–5) and regulation of protein function by phosphorylation and dephosphorylation (6). Several lines of evidence suggest that protein kinases and phosphatases play important roles in synaptic plasticity and memory (7–9). Protein phosphatase 2B (calcineurin, CaN), which is highly conserved from yeast to humans (10), is a Ca2+/calmodulin-activated protein phosphatase that has been implicated in synaptic plasticity and memory (9, 11, 12). For example, an increase in CaN activity produces deficits in long-term potentiation (LTP) and memory (13–15), and conversely, inhibition of CaN facilitates LTP and memory formation (16–20). However, other studies have shown that inhibition of CaN actually inhibits hippocampal LTP (21–24) and long-term facilitation in crayfish (25). Finally, mutant mice in which the regulatory subunit of CaN was knocked out in the adult forebrain show normal performance in contextual fear conditioning and the Morris water maze task, but show an impairment in working memory (26). Thus the mechanistic role of CaN in synaptic and behavioral plasticity remains to be fully elucidated.
An important step in understanding the role of CaN in the induction of plasticity is the identification of the molecular cascades that are under its regulatory control. One molecular pathway that has emerged as a major contributor to synaptic plasticity and memory in both vertebrates and invertebrates is the extracellular signal-regulated kinase/mitogen-activated protein kinase (MAPK) pathway (27). However, no functional link between CaN and the MAPK pathway in plasticity has been established. Here we demonstrate such a functional link by showing that CaN exerts a negative regulatory role on memory formation, at least in part through its actions on the MAPK signaling cascade.
Aplysia is well suited for the analysis of molecular mechanisms underlying different phases of memory, because these phases can be distinguished both temporally as well as mechanistically. Synaptic facilitation of the sensory-motor (SN-MN) synapses, a cellular model for sensitization in Aplysia, exists in at least three temporally and mechanistically different phases (28–31). One pulse of 5-hydroxytryptamine (5HT) (a modulatory neurotransmitter released in the CNS during sensitization training) (ref. 32, see also ref. 33) produces short-term facilitation (<30 min) of SN-MN synapses. This form of facilitation is independent of macromolecular synthesis. In contrast, five pulses of 5HT produce two temporally distinct phases of SN-MN facilitation: intermediate-term facilitation (>90 min) that requires translation but not transcription and long-term facilitation (>24 h) that requires translation as well as transcription (28, 29, 31). In parallel fashion, a single shock to the tail produces short-term memory (STM; <30 min) for sensitization of tail-elicited siphon withdrawal [T-SW (34, 35)] that does not require macromolecular synthesis (35). In contrast, five spaced tail shocks produce both intermediate-term memory (ITM; >90 min) that requires protein but not RNA synthesis and long-term memory (LTM; >24 h) that requires protein as well as RNA synthesis (35–37).
In this study, we examined the role of CaN in three different phases of memory for sensitization in Aplysia (STM, ITM, and LTM), by using the CaN inhibitor FK506. Because CaN has not yet been identified in Aplysia, when we refer to the target of FK506 as CaN it should be considered a “CaN-like” protein. We show that inhibition of CaN significantly facilitates the induction of both ITM and LTM with no effect on STM. Furthermore, we have identified a molecular cascade, the extracellular signal-regulated kinase 1/2/MAPK cascade, that contributes to the enhanced induction of ITM and LTM through CaN inhibition. Thus these results provide insights into the molecular mechanisms through which CaN exerts negative regulatory control on memory formation.
Materials and Methods
Phosphatase Assay.
A phosphatase assay was carried out by using a kit from Biomol (Plymouth Meeting, PA). Briefly, wild-caught Aplysia californica (250–400 g; supplied by Marinus, Long Beach, CA) were anesthetized by injecting isotonic MgCl2 (≈100 ml/100 g of body weight). The CNS (pleural, pedal, abdominal, cerebral, and buccal ganglia) was removed, and ganglia were desheathed in 50:50 MgCl2/artificial seawater (ASW) containing 460 mM NaCl, 55 mM MgCl2, 11 mM CaCl2, 10 mM KCl, 10 mM Tris, pH 7.6 to expose the neurons. The cells were washed with TBS (20 mM Tris, pH 7.5/150 mM NaCl), and extract was made. For the assay, the extract was diluted with buffer A (50 mM Tris, pH 7.5/0.2% Nonidet P-40/1 mM DTT/protease inhibitors) to obtain the readings in the linear range of phosphate determination and incubated with 2 mM CaCl2 and 62.5 μM FK506 (25 mM stock solution in DMSO, diluted 1:100 in buffer A before adding to the extract to a concentration of 62.5 μM) for 30 min at 30°C. For the control samples, an equivalent amount of DMSO was used. The reaction was started by adding the extract to the reaction mixture containing the RII phosphopeptide substrate. The final concentration of FK506 in the reaction mixture was 10 μM. After completion of the reaction and color development, absorbance (A) was measured at 620 nm. A620 values (after background subtraction) were taken as phosphatase activity. The activity sensitive to EGTA was taken as Ca2+-dependent phosphatase activity, and the activity remaining in the presence of EGTA was taken as Ca2+-independent activity. The Ca2+-dependent phosphatase activity in the presence of FK506 was obtained by subtracting the Ca2+-independent activity from total activity (both in the presence of FK506) and was expressed as percent of Ca2+-dependent phosphatase activity in the absence of FK506 (obtained by subtracting the Ca2+-independent activity from total activity, both in the absence of FK506).
Behavioral Procedures.
We used a reduced preparation for the behavioral experiments (Fig. 1A) that has been described in detail (35). Preparations were allowed at least 60 min to recover before pretest measurements. Before training, four pretests with an interstimulus interval of 15 min were conducted to measure baseline T-SW duration. In each of these tests, the right or left side of the tail (≈1 cm anterior from its tip, and midway between the lateral and medial margins) was stimulated with a brief jet of sea water (0.5-s duration). For sensitization training, tail shocks (1.5-s duration) were delivered through a bipolar hand-held electrode on the side used for the pretest measurements at an intertrial interval of 10 min. The nominal current across the electrode was 100 mA (ac), although most of this current is shunted by the seawater. Tests of T-SW after training (carried out in the same fashion as the pretests) were conducted at different times, depending on the phase of memory under examination.
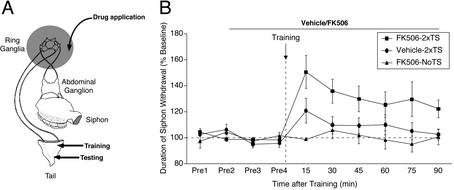
Figure 1.
Inhibition of CaN facilitates the induction of ITM for sensitization. (A) Schematic diagram of the reduced preparation used for behavioral experiments. (B) CaN inhibitor (FK506) or its vehicle was applied to the ring ganglia 30 min before training and was present throughout the testing period. Training was performed by using two spaced shocks (10-min intertrial interval) to the tail (2xTS). NoTS, control preparations not given any shock. Data are expressed as mean (±SEM) duration of T-SW normalized to baseline (FK506–2xTS, n = 9; vehicle-2xTS, n = 11; and FK506-NoTS, n = 4). In this and subsequent behavioral figures, horizontal dashed line denotes baseline T-SW and vertical dashed line denotes training.
The CaN inhibitor (FK506) was obtained from either Calbiochem or Biomol. Rapamycin and the MEK1/2 inhibitor (U0126) or its inactive analog (U0124) were obtained from Calbiochem. Stock solutions of the drugs were made in DMSO at 25 mM (FK506 and rapamycin) or 20 mM (U0126 and U0124). The inhibitors were diluted in ASW to 10 μM (FK506 and rapamycin) or 20 μM (U0126 and U0124) final concentration just before use. All drug treatments were restricted to the ring ganglia subchamber. The drug or vehicle (DMSO) incubations were carried out in static baths, which were exchanged manually. In all experiments, the control preparations received appropriate, equivalent bath exchanges.
In all experiments examining the effect of inhibiting CaN on memory for sensitization, FK506 or its vehicle was applied to the ring ganglia subchamber (after two pretests, five bath exchanges) for 30 min before training. Two additional pretests were taken at 15 and 30 min after beginning incubation with FK506/vehicle, after which the subchambers were exchanged with fresh solutions (two bath exchanges) just before training with two shocks to the tail (an additional 10-min incubation period was used in cases where a single shock was used for training). FK506/vehicle remained in the bath throughout testing in experiments examining STM and ITM or for 60 min after training in experiments examining LTM. The treatment with rapamycin was identical to that of FK506. For experiments examining the requirement of MAPK activity, the MEK inhibitor U0126, or its inactive analog U0124, was applied to the ring ganglia subchamber (five bath exchanges) 60 min before FK506 + U0126/FK506 + U0124 treatment (five bath exchanges, 30 min). Two additional pretests were taken during incubation with FK506 + U0126/FK506 + U0124. The drug baths were exchanged again just before training. U0126/U0124 remained in the bath throughout the duration of FK506 treatment. In experiments examining LTM for sensitization, FK506/U0126 were washed out of the subchamber 60 min after training, and preparations were perfused with cold (15–17°C) seawater overnight. Normal perfusion of the ring ganglia subchamber with ASW (room temperature) resumed 60 min before the 18-h test.
MAPK Activation.
Animals were anesthetized by injecting isotonic MgCl2 and pleural-pedal ganglia were removed. One pleural-pedal ganglia pair of an animal was assigned to control and the other pair was assigned to the experimental group. The ganglia were desheathed in 50:50 ASW/MgCl2 to expose the neurons, then washed with ASW by continuous perfusion for 15 min. To examine the effect of inhibition of CaN on MAPK activation, its specific inhibitor (FK506, 10 μM) or vehicle (0.04% DMSO) was applied by perfusion (dish volume ≈4 ml, flow rate ≈5 ml/min, five bath exchanges) and incubations were carried out for 30 min. To examine the effect of one pulse of 5HT and CaN inhibitor, the control ganglia were treated with vehicle for 25 min, 5HT was added to the bath to a final concentration of 50 μM, and incubations were carried out for an additional 5 min. The experimental ganglia were treated with FK506 for 25 min, and 5HT was added and incubated as described above. The pleural ganglia sensory clusters were excised immediately after the treatments and processed for Western blot analysis as described (38). The blots were first probed with anti-phospho-p42/44 MAPK antibody (which recognizes phosphorylated, active MAPK), stripped, and then probed with phospho-independent p42/44 MAPK antibody (which recognizes total MAPK). Horseradish peroxidase-conjugated anti-rabbit antibody was used as the secondary antibody and signal was detected by using the ECL reagent (Amersham Biosciences). The antibodies were obtained from Cell Signaling Technology (Beverly, MA). Exposure of the film was kept in the linear range and band intensity was quantified by using image software (National Institutes of Health, Bethesda, MD). Phospho-MAPK signal was normalized to the total MAPK signal in each sample (39, 40). Although the phospho-MAPK and phospho-independent MAPK antibodies recognize well-separated p42 and p44 in mammalian systems, consistent with earlier observations (41–43), we routinely observed a single band in Aplysia SN extracts. Occasionally however, we have observed two bands that run close together.
Data Analysis.
Duration of T-SW was measured by an observer who was blind to both training condition and drug treatment. The duration of T-SW was defined as the elapsed time from stimulus onset to the initial relaxation of the siphon from the contracted position (35). In all experiments, baseline duration of T-SW was determined by the average of the pretests.
For statistical analysis, differences among groups were examined with factorial ANOVA followed by Fisher's protected least significant difference post hoc test. Differences within a group were assessed with a paired t test. For MAPK activation, the raw phospho-MAPK/total MAPK ratios of experimental and control groups were used, and for the phosphatase assay, A620 values were used for statistical evaluation.
Results
Inhibition of CaN Facilitates the Induction of ITM for Sensitization.
To examine the role of CaN in different phases of memory in Aplysia, it was first necessary to determine that FK506, a commonly used CaN inhibitor, inhibited phosphatase activity in Aplysia. We found that there was a significant reduction in Ca2+-dependent phosphatase activity (but no change in total Ca2+-independent phosphatase activity) when Aplysia CNS extracts were treated with FK506 [Ca2+-dependent, 78.04 ± 3.35%, t3 = −5.61, P < 0.05; Ca2+-independent, 103.02 ± 2.74%, t3 = 1.13, not significant (NS)]. Thus FK506 inhibits Ca2+-dependent phosphatase activity in Aplysia.
We next asked whether FK506 had any effect on the induction of ITM in Aplysia. We used a reduced preparation (Fig. 1A, ref. 35) in which pharmacological agents can be selectively applied to the ring ganglia, while simultaneously measuring T-SW. We used a training regimen (two spaced tail shocks) that is known to be insufficient to induce ITM (44). FK506 (10 μM; refs. 45 and 46), which binds to intracellular FK-binding protein (FKBP12) and inhibits CaN activity (10, 47), was applied to the ring ganglia subchamber 30 min before training and was present throughout the testing period. Four groups were examined: (i) two tail shocks + vehicle, (ii) two tail shocks + FK506, (iii) FK506 alone (no tail shocks), and (iv) two tail shocks + rapamycin (a negative control for FK506). As shown in Fig. 1B, two tail shocks alone did not induce significant ITM for sensitization (90 min, t10 = 0.49, NS). In contrast, significant ITM was induced with the same subthreshold training when CaN was inhibited (90 min, t8 = 2.94, P < 0.05). Importantly, the same course of treatment with FK506 had no effect on baseline T-SW in nontrained control preparations examined in parallel (90 min, t3 = −0.11, NS). In addition, rapamycin, which binds to FKBP12 but does not inhibit the enzymatic activity of CaN (48), and thus can serve as an effective negative control for FK506 (45, 46, 49) had no effect on the induction of ITM (data not shown, 90 min, 92.01 ± 4.91%, t2 = −1.65, NS), indicating that the effect of FK506 was likely caused by inhibition of CaN, although other possible targets of FK506 cannot be completely ruled out. A between-group comparison revealed a significant difference among groups (90 min, F3,23 = 4.12, P < 0.05). A posthoc analysis revealed a significant enhancement of T-SW in trained preparations treated with FK506 relative to vehicle-treated/trained, rapamycin-treated/trained, and FK506-treated/nontrained preparations (P < 0.05). These results demonstrate that CaN inhibition significantly facilitates the induction of ITM for sensitization in Aplysia.
CaN Inhibition Facilitates MAPK Activation.
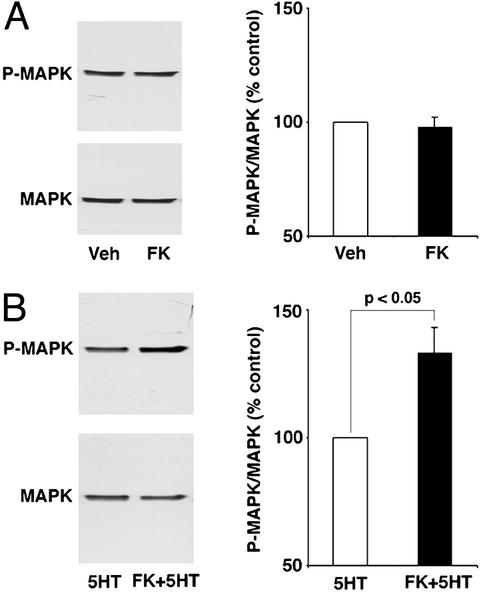
We have recently shown that the normal induction of ITM with five tail shocks (35) requires MAPK activity (50). Given this observation, we were interested in asking whether the MAPK cascade was involved in the facilitated ITM induced by CaN inhibition. To examine this question, we first asked whether MAPK was activated with CaN inhibition in conjunction with a subthreshold application of 5HT, a modulatory neurotransmitter released in the CNS during sensitization training (ref. 32 and unpublished observations). Desheathed pleural-pedal ganglia were exposed to FK506 for 30 min in the presence or absence of a single pulse of 5HT, and MAPK activation was examined by using phospho-dependent and phospho-independent MAPK antibodies. Two control groups were examined in parallel, one was treated with vehicle, and the other with a single pulse of 5HT. Confirming a previous report (41), a single pulse of 5HT had no effect on MAPK activation (NS, data not shown). Moreover, CaN inhibition alone also had no effect on MAPK activation (97.74 ± 4.58%, t11 = −0.46, NS) (Fig. 2A). However, there was significant activation of MAPK when FK506 application was paired with one pulse of 5HT (133.11 ± 9.99%, t17 = 3.12, P < 0.05) (Fig. 2B). These results indicate that CaN inhibition reduces the threshold for 5HT-induced MAPK activation. The dual phosphorylation of MAPK is necessary and sufficient for MAPK activation and the increased phosphorylation of MAPK we observe (Fig. 2B) is commonly taken as a reliable measure of MAPK activation (39, 40, 43).
Figure 2.
Inhibition of CaN facilitates MAPK activation by 5HT. (A) Pleural-pedal ganglia were treated with either vehicle (Veh) or FK506 (FK) for 30 min, and MAPK activation was examined in the SNs (n = 12 per group) by using phospho-dependent and total MAPK antibodies. (B) Pleural-pedal ganglia were treated with either vehicle and one pulse of 5HT (5HT) or one pulse of 5HT coterminating with 30-min FK506 application (FK + 5HT), and MAPK activation was examined in the SNs (n = 18 per group). Data are presented as mean ± SEM (percentage of control). Shown are the sample blots with phospho-MAPK and total MAPK antibodies (Left) and the summary data (Right).
MAPK Activity Is Required for the Facilitation of ITM Induced by CaN Inhibition.
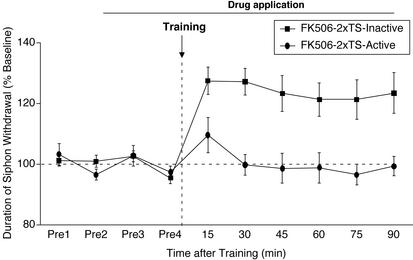
The fact that the induction of ITM and LTM requires MAPK activity (50), together with the observation that inhibition of CaN facilitates 5HT-induced MAPK activation (Fig. 2B), suggested that MAPK activity may be required for the facilitation of ITM produced by CaN inhibition (Fig. 1B). We examined the role of MAPK in facilitated ITM by inhibiting MEK1/2, a kinase that acts upstream of MAPK and activates it. We used the compound U0126, a specific MEK inhibitor (51) that has been shown to block MAPK activation in Aplysia (42, 43). The MEK inhibitor (U0126, 20 μM) or its inactive analog (U0124, 20 μM) was applied to the ganglia subchamber 60 min before the application of FK506 (30 min, with U0126/U0124), and FK506/U0126 or FK506/U0124 were present throughout the testing period. Three groups were examined: (i) two tail shocks + U0124 + FK506, (ii) two tail shocks + U0126 + FK506, and (iii) U0126 alone to assess its effects on baseline T-SW. As shown in Fig. 3, whereas significant facilitated ITM was induced in trained preparations treated with U0124 (90 min, t7 = 3.45, P < 0.05), ITM was abolished in trained preparations treated with U0126 (90 min, t7 = −0.3, NS). The same course of treatment with U0126 had no effect on baseline T-SW in nontrained control preparations (data not shown, 90 min, 95.78 ± 3.63%, t3 = −1.18, NS). A between-group comparison revealed a significant difference between the groups (90 min, F2,17 = 8.1, P < 0.05). A posthoc analysis showed a significant enhancement of T-SW in trained preparations treated with U0124/FK506 relative to the trained preparations treated with U0126/FK506 and the U0126-treated/nontrained preparations (P < 0.05). These results show that MAPK activity is necessary for the facilitation of ITM induced by inhibition of CaN.
Figure 3.
Facilitated ITM induced by CaN inhibition requires MAPK activity. The MEK inhibitor (U0126, Active) or its inactive analog (U0124, Inactive) was applied to the ring ganglia for 60 min, after which FK506 along with U0126/U0124 was applied for 30 min before training; both were present throughout the testing period. Training was performed by using two spaced shocks to the tail (2xTS). Data are expressed as mean (±SEM) duration of T-SW normalized to baseline (FK506–2xTS-Inactive, n = 8; FK506–2xTS-Active, n = 8).
CaN Inhibition Does Not Affect STM for Sensitization.
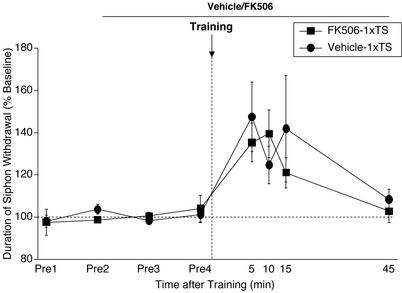
It is clear from the results shown in Fig. 1B that memory at the 15-min test after two tail shocks (which might be considered STM) was enhanced in preparations treated with the CaN inhibitor. This finding raised the possibility that STM for sensitization is also regulated by CaN inhibition. To examine this idea directly, we analyzed STM induced by a single tail shock (35). The CaN inhibitor was applied to the ganglia subchamber 40 min before training and was present throughout the testing period. The results shown in Fig. 4 demonstrate that STM for sensitization was not affected by CaN inhibition (5–15 min, all F1,11 < 1.1, NS). The reason for the enhancement of memory for sensitization at 15 min induced by two tail shocks and CaN inhibition (Fig. 1B) remains to be investigated. One possibility is that the enhanced memory tested at 15 min after two shocks/FK506 treatment actually reflects the beginning of the ITM process.
Figure 4.
STM for sensitization is unaffected by CaN inhibition. The CaN inhibitor (FK506) or its vehicle was applied 40 min before training and was present throughout the testing period. Training was performed by using a single shock to the tail (1xTS). Data are expressed as mean (± SEM) duration of T-SW normalized to baseline (FK506–1xTS, n = 6 and Vehicle-1xTS, n = 7).
CaN Inhibition Facilitates the Induction of LTM for Sensitization in a MAPK-Dependent Fashion.
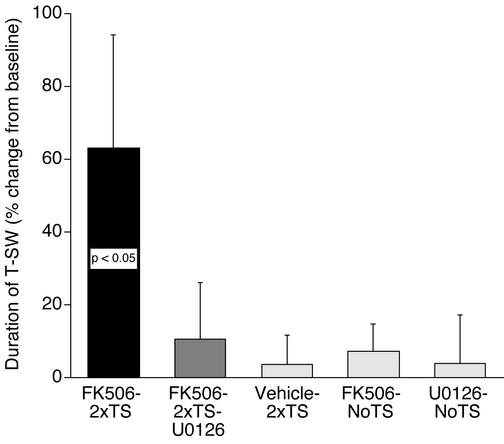
In a final series of experiments, we asked whether CaN inhibition had a facilitatory effect on the induction of LTM, and if so, whether such facilitated LTM required MAPK activity. FK506 was applied 30 min before training and was present until 1 h after training. The MEK inhibitor (U0126) was applied 60 min before the application of FK506 + U0126 (30 min) and both were present until 1 h after training. Training was performed with two tail shocks, which are known to be insufficient for inducing LTM (43, 44). Five groups were examined: (i) two tail shocks + vehicle, (ii) two tail shocks + FK506, (iii) two tail shocks + U0126 + FK506, (iv) FK506 alone, and (v) U0126 alone. These last two groups were run to assess effects on baseline T-SW. As shown in Fig. 5, two tail shocks alone did not induce LTM (t6 = 0.28, NS). In contrast, significant LTM was induced by the same subthreshold training when combined with CaN inhibition (t8 = 1.89, P < 0.05, one tail). The same course of treatment with FK506 had no effect on baseline T-SW at the long-term test in nontrained preparations (t4 = 0.97, NS). Importantly, like ITM, facilitated induction of LTM by CaN inhibition was blocked by U0126 (t8 = 0.53, NS). Finally, the MEK inhibitor had no effect on baseline T-SW at the long-term test in nontrained preparations (t3 = −0.05, NS). These results demonstrate two important conclusions: (i) CaN inhibition significantly facilitates the induction of LTM for sensitization, and (ii) the facilitatory effect of CaN inhibition on LTM formation requires MAPK activity.
Figure 5.
CaN inhibition facilitates the induction of LTM for sensitization in a MAPK-dependent manner. The MEK inhibitor (U0126) or its vehicle was applied to the ring ganglia for 60 min, then FK506 (alone or with U0126) was applied 30 min before training, which was performed by using two spaced shocks to the tail (2xTS). The drugs were present until 60 min after training. Data are expressed as mean (±SEM) duration of T-SW 18 h after training (percentage change from baseline, FK506–2xTS, n = 9; FK506–2xTS-U0126, n = 9; Vehicle-2xTS, n = 7; FK506-NoTS, n = 5; U0126-NoTS, n = 4). NoTS, control preparations not given any shock. The enhancement of LTM by CaN inhibition is blocked by inhibition of MAPK.
Discussion
A number of studies suggest that protein kinases and phosphatases play important regulatory roles in synaptic plasticity and memory formation (7–9). In this study, we show that FK506, a specific CaN inhibitor, inhibits Ca2+-dependent phosphatase activity in Aplysia neuronal extracts. Although significant inhibition was observed, it was modest. However, a relatively small change in an early step of a molecular cascade can have profound effects on the final outcome of a complex function such as memory formation. We further show that inhibition of CaN, or at least a CaN-like protein in Aplysia, facilitates the induction of ITM and LTM, suggesting that CaN acts as an inhibitory constraint in the formation of long-lasting memories in this system. Our observations are consistent with previous studies reporting that CaN can constrain LTP and memory formation. In the hippocampus, cytosolic activity and expression levels of CaN increase with age, which is correlated with deficits in memory (52). Mice that overexpress a truncated form of CaN have deficits in LTP (13) and impaired LTM without any effect on STM (14). Conversely, reduction in CaN activity by antisense oligonucleotides reduces the threshold for LTP induction and facilitates memory (17, 18). Similarly, expression of a CaN inhibitor in the mouse brain facilitates the induction of LTP in vitro and in vivo and leads to enhanced learning and memory (20). However, other studies suggest that CaN may not act as a general negative constraint in all cases and may in fact have a positive role in the induction of plasticity and memory (21–26). Thus the role of CaN in synaptic plasticity and memory is not yet fully understood.
The observations described above raise the important question as to the molecular cascades that are regulated by CaN. We have identified one such pathway, the MAPK cascade, that is required for the facilitated induction of long-lasting phases of memory with CaN inhibition. Previous results in Aplysia have shown that repeated pulses of 5HT activate MAPK in the SNs, but a single pulse is insufficient for MAPK activation (41). We found that a single pulse of 5HT becomes sufficient to induce MAPK activation when it is applied in the presence of a CaN inhibitor. Collectively, these observations show that one molecular cascade that is under inhibitory control by CaN is the MAPK cascade, which is known to play important roles in the induction of long-lasting facilitation at the SN-MN synapse (43, 53) and long-lasting memory in Aplysia (50). Our observations further indicate that the effect of CaN inhibition on memory formation in Aplysia is upstream of MAPK, because the facilitated ITM and LTM induced by CaN inhibition is abolished when MEK, an upstream kinase that phosphorylates and activates MAPK, is inhibited in the CNS.
We used 5HT to examine the activation of MAPK, whereas tail shocks were used to examine sensitization in the behavioral experiments. The rationale for this approach is as follows: we have previously shown that both tail-nerve shock and tail shock induce the release of 5HT in the CNS (ref. 32 and unpublished data). In addition, application of 5HT both to the intact CNS and SN-MNs in culture has served as an analog of sensitization training in a wide variety of experiments (28–31, 53). Finally, the tail shock procedure that we used, which alone does not induce ITM or LTM (Figs. 1 and 5), also does not induce MAPK activation when examined 1 h after tail shocks (J. Shobe and T.J.C., unpublished observations). Thus, the observation that the facilitated induction of ITM and LTM by FK506 requires MAPK activity (Figs. 3 and 5) strongly supports the view that FK506 enhances MAPK activation by two tail shocks.
What downstream molecular steps might be regulated by facilitated MAPK activation induced by CaN inhibition? Because ITM requires translation but not transcription (35), inhibition of CaN, leading to 5HT-induced activation of MAPK, could regulate aspects of protein synthesis that are required for the induction of ITM. Consistent with this hypothesis, Takei et al. (54) recently showed that brain-derived neurotrophic factor-induced protein synthesis requires MAPK activity. In addition to protein synthesis, the facilitatory effect of CaN inhibition in the induction of LTM is likely to involve the regulation of transcription factors and possibly cytoplasmic substrates (55). Among the transcription factors, cAMP response element-binding protein (CREB), C/EBP, and Elk-1 are potential candidates. Phosphorylation of CREB and subsequent cAMP response element-dependent gene expression plays important roles in synaptic plasticity and memory (56, 57). In Aplysia, MAPK phosphorylates CREB2 (a repressor of CREB 1a) and C/EBP (41). Phosphorylation of C/EBP leads to an increase in its DNA binding activity (58). In principle, CaN inhibition could facilitate CREB-dependent transcription in two ways: (i) by MAPK-dependent CREB activation (through p90rsk, refs. 59 and 60) and/or MAPK-dependent relief of repression by CREB2 (41, 53), and (ii) by inhibition of protein phosphatase 1 (PP1) through protein phosphatase inhibitor-1 (6). PP1 is known to dephosphorylate and regulate CREB activation (61, 62) and has recently been shown to act as a negative constraint in learning and memory (63). Consistent with this overall view, inhibition of CaN has been shown to produce sustained CREB phosphorylation by weak synaptic input that normally produces only transient CREB phosphorylation (62). In addition, CaN dephosphorylates Elk1, a transcription factor acting downstream of MAPK (64). Thus, in considering its facilitatory role in LTM formation, CaN inhibition may act to enhance transcription in two ways: first, by stimulating transcription through MAPK activation, and second, by preventing dephosphorylation of CREB and Elk1, thereby prolonging the activation state of these transcription factors.
In conclusion, we have shown that inhibition of CaN facilitates the induction of ITM and LTM in Aplysia, without any effect on STM, and that inhibition of CaN also facilitates MAPK activation by subthreshold application of 5HT. Finally, we show that the facilitatory effect of CaN inhibition on the induction of ITM and LTM depends on MAPK activity. These results indicate that CaN acts as an important inhibitory constraint on the formation of long-lasting phases of memory for sensitization in this system, and that at least one molecular pathway regulated by CaN is the MAPK cascade. It will now be of interest to examine whether CaN activity is modulated by the stimuli that are normally required to produce both long-lasting synaptic facilitation and long-lasting memory for sensitization.
Acknowledgments
We thank Angela Purcell, Adam Bristol, and Carolyn Sherff for critically reading an earlier version of this manuscript and Justin Shobe for helpful discussions. This work was supported by National Science Foundation Grant IBN-0049013 and National Institute of Mental Health Grant RO1-MH-14-1083 (to T.J.C.).
Abbreviations
- MAPK
mitogen-activated protein kinase
- CaN
calcineurin
- CREB
cAMP response element-binding protein
- LTP
long-term potentiation
- SN-MN
sensory-motor
- 5-HT
5-hydroxytryptamine
- STM
short-term memory
- T-SW
tail-elicited siphon withdrawal
- ITM
intermediate-term memory
- LTM
long-term memory
- ASW
artificial seawater
- NS
not significant
References
- 1.Abel T, Martin K C, Bartsch D, Kandel E R. Science. 1998;279:338–341. doi: 10.1126/science.279.5349.338. [DOI] [PubMed] [Google Scholar]
- 2.Bartsch D, Ghirardi M, Skehel P A, Karl K A, Herder S P, Chen M, Baily C H. Cell. 1995;83:979–992. doi: 10.1016/0092-8674(95)90213-9. [DOI] [PubMed] [Google Scholar]
- 3.Bartsch D, Casadio A, Karl K A, Serodio P, Kandel E R. Cell. 1998;95:211–223. doi: 10.1016/s0092-8674(00)81752-3. [DOI] [PubMed] [Google Scholar]
- 4.Yin J C, Wallach J S, Del Vecchio M, Wilder E L, Zhou H, Quinn W G, Tully T. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- 5.Yin J C, Del Vecchio M, Zhou H, Tully T. Cell. 1995;81:107–115. doi: 10.1016/0092-8674(95)90375-5. [DOI] [PubMed] [Google Scholar]
- 6.Lisman J. Proc Natl Acad Sci USA. 1989;86:9574–9578. doi: 10.1073/pnas.86.23.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Micheau J, Riedel G. Cell Mol Life Sci. 1999;55:534–548. doi: 10.1007/s000180050312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soderling T R, Derkach V A. Trends Neurosci. 2000;23:75–80. doi: 10.1016/s0166-2236(99)01490-3. [DOI] [PubMed] [Google Scholar]
- 9.Winder D G, Sweatt J D. Nat Rev Neurosci. 2001;2:461–474. doi: 10.1038/35081514. [DOI] [PubMed] [Google Scholar]
- 10.Hemenway C S, Heitman J. Cell Biochem Biophys. 1999;30:115–151. doi: 10.1007/BF02737887. [DOI] [PubMed] [Google Scholar]
- 11.Yakel J L. Trends Pharmacol Sci. 1997;18:24–34. doi: 10.1016/s0165-6147(97)01046-8. [DOI] [PubMed] [Google Scholar]
- 12.Sugiura R, Sio S O, Shuntoh H, Kuno T. Cell Mol Life Sci. 2001;58:278–288. doi: 10.1007/PL00000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winder D G, Mansuy I M, Osman M, Moallem T M, Kandel E R. Cell. 1998;92:25–37. doi: 10.1016/s0092-8674(00)80896-x. [DOI] [PubMed] [Google Scholar]
- 14.Mansuy I M, Mayford M, Jacob B, Kandel E R, Bach M E. Cell. 1998;92:39–49. doi: 10.1016/s0092-8674(00)80897-1. [DOI] [PubMed] [Google Scholar]
- 15.Mansuy I M, Winder D G, Moallem T M, Osman M, Mayford M, Hawkins R D, Kandel E R. Neuron. 1998;21:257–265. doi: 10.1016/s0896-6273(00)80533-4. [DOI] [PubMed] [Google Scholar]
- 16.Funauchi M, Haruta H, Tsumoto T. Neurosci Res. 1994;19:269–278. doi: 10.1016/0168-0102(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 17.Ikegami S, Kato A, Kudo Y, Kuno T, Ozawa F, Inokuchi K. Brain Res Mol Brain Res. 1996;41:183–191. doi: 10.1016/0169-328x(96)00094-0. [DOI] [PubMed] [Google Scholar]
- 18.Ikegami S, Inokuchi K. Neuroscience. 2000;98:637–646. doi: 10.1016/s0306-4522(00)00161-5. [DOI] [PubMed] [Google Scholar]
- 19.Wang J H, Kelly P T. Learn Mem. 1996;3:170–181. doi: 10.1101/lm.3.2-3.170. [DOI] [PubMed] [Google Scholar]
- 20.Malleret G, Haditsch U, Genoux D, Jones M W, Bliss T V, Vanhoose A M, Weitlauf C, Kandel E R, Winder D G, Mansuy I M. Cell. 2001;104:675–686. doi: 10.1016/s0092-8674(01)00264-1. [DOI] [PubMed] [Google Scholar]
- 21.Wang J H, Stelzer A. NeuroReport. 1994;5:2377–2380. doi: 10.1097/00001756-199411000-00041. [DOI] [PubMed] [Google Scholar]
- 22.Lu Y F, Hayashi Y, Moriwaki A, Tomizawa K, Matsui H. Neurosci Lett. 1996;205:103–106. doi: 10.1016/0304-3940(96)12384-3. [DOI] [PubMed] [Google Scholar]
- 23.Lu Y F, Tomizawa K, Moriwaki A, Hayashi Y, Tokuda M, Itano T, Hatase O, Matsui H. Brain Res. 1996;729:142–146. [PubMed] [Google Scholar]
- 24.Onuma H, Lu Y F, Tomizawa K, Moriwaki A, Tokuda M, Hatase O, Matsui H. Neurosci Res. 1998;30:313–319. doi: 10.1016/s0168-0102(98)00012-1. [DOI] [PubMed] [Google Scholar]
- 25.Beaumont V, Zhong N, Fletcher R, Froemke R C, Zucker R S. Neuron. 2001;32:489–501. doi: 10.1016/s0896-6273(01)00483-4. [DOI] [PubMed] [Google Scholar]
- 26.Zeng H, Chattarji S, Barbarosie M, Rondi-Reig L, Philpot B D, Miyakawa T, Bear M F, Tonegawa S. Cell. 2001;107:617–629. doi: 10.1016/s0092-8674(01)00585-2. [DOI] [PubMed] [Google Scholar]
- 27.Adams J P, Sweatt J D. Annu Rev Pharmacol Toxicol. 2002;42:135–163. doi: 10.1146/annurev.pharmtox.42.082701.145401. [DOI] [PubMed] [Google Scholar]
- 28.Montarolo P G, Goelet P, Castellucci V F, Morgan J, Kandel E R, Schacher S. Science. 1986;234:1249–1254. doi: 10.1126/science.3775383. [DOI] [PubMed] [Google Scholar]
- 29.Ghirardi M, Montarolo P G, Kandel E R. Neuron. 1995;14:413–420. doi: 10.1016/0896-6273(95)90297-x. [DOI] [PubMed] [Google Scholar]
- 30.Mauelshagen J, Parker G R, Carew T J. J Neurosci. 1996;16:7099–7108. doi: 10.1523/JNEUROSCI.16-22-07099.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutton M A, Carew T J. Neuron. 2000;26:219–231. doi: 10.1016/s0896-6273(00)81152-6. [DOI] [PubMed] [Google Scholar]
- 32.Marinesco S, Carew T J. J Neurosci. 2002;22:2299–2312. doi: 10.1523/JNEUROSCI.22-06-02299.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levenson J, Byrne J H, Eskin A. J Neurosci. 1999;19:8094–8103. doi: 10.1523/JNEUROSCI.19-18-08094.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldsmith J R, Byrne J H. J Neurosci. 1993;13:1688–1700. doi: 10.1523/JNEUROSCI.13-04-01688.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutton M A, Masters S E, Bagnall M W, Carew T J. Neuron. 2001;31:143–154. doi: 10.1016/s0896-6273(01)00342-7. [DOI] [PubMed] [Google Scholar]
- 36.Levenson J, Endo S, Kategaya L S, Fernandez R I, Brabham D G, Chin J, Byrne J H, Eskin A. Proc Natl Acad Sci USA. 2000;97:12858–12863. doi: 10.1073/pnas.220256497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castellucci V F, Blumenfeld H, Goelet P, Kandel E R. J Neurobiol. 1989;20:1–9. doi: 10.1002/neu.480200102. [DOI] [PubMed] [Google Scholar]
- 38.Sharma S K, Carew T J. Anal Biochem. 2002;307:187–189. doi: 10.1016/s0003-2697(02)00008-8. [DOI] [PubMed] [Google Scholar]
- 39.Patterson S L, Pittenger C, Morozov A, Martin K C, Scanlin H, Drake C, Kandel E R. Neuron. 2001;32:123–140. doi: 10.1016/s0896-6273(01)00443-3. [DOI] [PubMed] [Google Scholar]
- 40.Mazzucchelli C, Vantaggiato C, Ciamei A, Fasano S, Pakhotin P, Krezel W, Welzl H, Wolfer D P, Pagès G, Valverde O, et al. Neuron. 2002;34:807–820. doi: 10.1016/s0896-6273(02)00716-x. [DOI] [PubMed] [Google Scholar]
- 41.Michael D, Martin K C, Seger R, Ning M M, Baston R, Kandel E R. Proc Natl Acad Sci USA. 1998;95:1864–1869. doi: 10.1073/pnas.95.4.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chin J, Angers A, Cleary L J, Eskin A, Byrne J H. J Neurosci. 2002;22:RC220. doi: 10.1523/JNEUROSCI.22-09-j0004.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Purcell A L, Sharma S K, Bagnall M W, Sutton M A, Carew T J. Neuron. 2003;37:473–484. doi: 10.1016/s0896-6273(03)00030-8. [DOI] [PubMed] [Google Scholar]
- 44.Sutton M A, Ide J, Masters S E, Carew T J. Learn Mem. 2002;9:29–40. doi: 10.1101/lm.44802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu F C, Graybiel A M. Neuron. 1996;17:1133–1144. doi: 10.1016/s0896-6273(00)80245-7. [DOI] [PubMed] [Google Scholar]
- 46.Li S T, Kato K, Tomizawa K, Matsushita M, Moriwaki A, Matsui H, Mikoshiba K. J Neurosci. 2002;22:5034–5041. doi: 10.1523/JNEUROSCI.22-12-05034.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dumont F J. Curr Med Chem. 2000;7:731–748. doi: 10.2174/0929867003374723. [DOI] [PubMed] [Google Scholar]
- 48.Hashimoto Y, Perrino B A, Soderling T R. J Biol Chem. 1990;265:1924–1927. [PubMed] [Google Scholar]
- 49.Lu Y M, Mansuy I M, Kandel E R, Roder J. Neuron. 2000;26:197–205. doi: 10.1016/s0896-6273(00)81150-2. [DOI] [PubMed] [Google Scholar]
- 50. Sharma, S. K., Sherff, C. M., Shobe, J., Bagnall, M. W., Sutton, M. A. & Carew, T. J. (2003) J. Neurosci., in press. [DOI] [PMC free article] [PubMed]
- 51.DeSilva D R, Jones E A, Favata M F, Jafee B D, Magdela R L, Trzaskos J M, Scherle P A. J Immunol. 1998;160:4175–4181. [PubMed] [Google Scholar]
- 52.Foster T C, Sharrow K M, Masse J R, Norris C M, Kumar A. J Neurosci. 2001;21:4066–4073. doi: 10.1523/JNEUROSCI.21-11-04066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin K C, Michael D, Rose J C, Barad M, Casadio A, Zhu H, Kandel E R. Neuron. 1997;18:899–912. doi: 10.1016/s0896-6273(00)80330-x. [DOI] [PubMed] [Google Scholar]
- 54.Takei N, Kawamura M, Hara K, Yonezawa K, Nawa H. J Biol Chem. 2001;276:42818–42825. doi: 10.1074/jbc.M103237200. [DOI] [PubMed] [Google Scholar]
- 55.Bailey C H, Kang B K, Chen M, Martin K C, Lim C S, Casadio A, Kandel E R. Neuron. 1997;18:913–924. doi: 10.1016/s0896-6273(00)80331-1. [DOI] [PubMed] [Google Scholar]
- 56.Dash P K, Hochner B, Kandel E R. Nature. 1990;345:718–721. doi: 10.1038/345718a0. [DOI] [PubMed] [Google Scholar]
- 57.Silva A J, Kogan J H, Frankland P W, Kida S. Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- 58.Yamamoto N, Hegde A N, Chain D G, Schwartz J H. J Neurochem. 1999;73:2415–2423. doi: 10.1046/j.1471-4159.1999.0732415.x. [DOI] [PubMed] [Google Scholar]
- 59.Xing J, Ginty D D, Greenberg M E. Science. 1996;273:959–963. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]
- 60.Impey S, Obrietan K, Wong S T, Poser S, Yano S, Wayman G, Deloulme J C, Chan G, Storm D R. Neuron. 1998;21:869–883. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 61.Hagiwara M, Alberts A, Brindle P, Meinkoth J, Feramisco J, Deng T, Karin M, Shenolikar S, Montminy M. Cell. 1992;70:105–113. doi: 10.1016/0092-8674(92)90537-m. [DOI] [PubMed] [Google Scholar]
- 62.Bito H, Deisseroth K, Tsien R W. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- 63.Genoux D, Haditsch U, Knobloch M, Michalon A, Storm D, Mansuy I M. Nature. 2002;418:970–975. doi: 10.1038/nature00928. [DOI] [PubMed] [Google Scholar]
- 64.Sugimoto T, Stewart S, Guan K L. J Biol Chem. 1997;272:29415–29418. doi: 10.1074/jbc.272.47.29415. [DOI] [PubMed] [Google Scholar]