Abstract
De novo jasmonic acid (JA) synthesis is required for wound-induced expression of proteinase inhibitors and other defense genes in potato and tomato. The first step in JA biosynthesis involves lipoxygenase (LOX) introducing molecular oxygen at the C-13 position of linolenic acid. We previously have shown that, in potato, at least two gene families code for 13-LOX proteins. We have now produced transgenic potato plants devoid of one specific 13-LOX isoform (LOX-H3) through antisense-mediated depletion of its mRNA. LOX-H3 depletion largely abolishes accumulation of proteinase inhibitors on wounding, indicating that this specific LOX plays an instrumental role in the regulation of wound-induced gene expression. As a consequence, weight gain of Colorado potato beetles fed on antisense plants is significantly larger than those fed on wild-type plants. The poorer performance of LOX-H3-deficient plants toward herbivory is more evident with a polyphagous insect; larvae of beet armyworm reared on the antisense lines have up to 57% higher weight than those fed on nontransformed plants. LOX-H3 thus appears to regulate gene activation in response to pest attack, and this inducible response is likely to be a major determinant for reducing performance of nonspecialized herbivores. However, the regulatory role of LOX-H3 is not caused by its involvement in the wound-induced increase of JA, as wild-type and LOX-H3 deficient plants have similar jasmonate levels after wounding. LOX-H3-deficient plants have higher tuber yields. The apparent effect of suppressing the inducible defensive response on plant vigor suggests that it may pose a penalty in plant fitness under nonstress situations.
In addition to constitutive barriers, plants have acquired inducible defenses to react to pest attack, which do not seem to compromise plant fitness as severely as a constitutively active defense system (1). On their side, a majority of insects are very specific for the plant species and the parts of plants they feed on (2). Inducible mechanisms in plants largely rely on transcriptional activation of defensive genes (3). Although, in some cases, inducing pathways may specifically require insect–plant interaction (4), others are activated by the damage caused by chewing and are thus also inducible by mechanical wounding, as those leading to the accumulation of proteinase inhibitors (5).
The wound-induced synthesis of the plant hormone jasmonic acid (JA) is one of the last steps in the signaling pathway leading to activation of wound-responsive genes, those encoding proteinase inhibitors among others (6, 7, 8). Several components of this wound-signaling pathway have been identified in potato and tomato (9). Wound-responsive gene activation on treatment with plant cell wall-derived oligosaccharides (10), the peptide systemin (11), or the plant hormone abscisic acid (12) has, in all cases, been shown to be mediated by an elevation in the endogenous levels of JA caused by its de novo synthesis. Interfering with this signaling pathway, by antisense-mediated depletion of systemin (13) or mutations in genes involved in abscisic acid (14) or JA biosynthesis (11), results in reduced accumulation of defensive gene transcripts after wounding. In addition, in the cases tested, the altered plants are more susceptible to pest attack (11, 15), suggesting that inducible defense systems play, indeed, an instrumental role in plant–pest interactions. The involvement of JA in the defensive response of Arabidopsis thaliana to pest attack also has been demonstrated (16).
JA is a derivative of linolenic acid, a major fatty acid constituent of plant membrane lipids, from which it is released on wounding (17). Lipoxygenases (LOX) are thought to act on free unsaturated fatty acids, introducing molecular oxygen in a stereospecific manner to give rise, in the case of linolenic acid, to its 9- and 13-hydroperoxides (18). While 9-hydroperoxides subsequently are converted to aldehydes and oxoacids that may have antimicrobial activities, the 13-hydroperoxy linolenic acid can be converted to either oxoacids and aldehydes after hydroperoxide lyase-catalyzed cleavage or JA after enzymatic cyclation, reduction, and β-oxidation (19). Cosupression-mediated depletion of a specific LOX isoform in A. thaliana led to a decrease in the wound-induced JA levels whereas basal levels remained largely unaffected (20), suggesting that an alternative pathway could exist for basal JA synthesis. LOX also may use linoleic acid and other unsaturated fatty acids as substrates, giving rise to a series of oxylipin compounds that also may affect gene expression (21, 22).
LOX are thus a complex array of distinct enzymes playing regulatory roles in the production of defense-related compounds. Different LOX isoforms usually are encoded in complex, multigene families (23). Antisense-mediated LOX depletion has proved an elegant approach for elucidating the involvement of a specific LOX isoform in the incompatibility trait of a tobacco variety resistant to the fungus Phytophtora parasitica (24). In potato, at least three LOX gene families have been characterized, one encoding a 9-LOX mainly expressed in tubers and roots (25, 26), and two others encoding different 13-LOX isoforms that are induced in the leaves in response to wounding (26). We have used the antisense approach to elucidate the involvement of the identified LOX genes in JA synthesis. The performance of insects feeding on LOX-depleted plants has been established by using two different species: a leaf-feeding specialist of solanaceous plants, Colorado potato beetle (Leptinotarsa decemlineata), and the beet armyworm (Spodoptera exigua), which as a generalist species might be more sensitive to specific wound-induced chemical changes. Because, in addition to its role in plant defense, JA has been suggested to be a tuber-inducing factor in potato (27), we have determined the tuber yields of these transgenic plants.
MATERIALS AND METHODS
Plant Materials and Transformation.
Potato plants (Solanum tuberosum cv. Desiree) were grown in soil in the greenhouse at 23°C, under a 16-h light/8-h darkness regime. Plants were transformed as described (28) by cocultivation with an Agrobacterium tumefaciens strain harboring in the BIN19 vector (29) the full length LOX-H3 cDNA (26) in antisense orientation under the control of the cauliflower mosaic virus (CaMV) 35S promoter and the octopine synthase gene 3′ terminator sequences (30). The fidelity of the construct in A. tumefaciens was tested by Southern hybridization and sequencing of the promoter-cDNA and cDNA-3′ terminator junctions (not shown). Plant transformants were selected for resistance to 50 mg/liter kanamycin (Sigma) and, after rooting, were transferred to soil and were grown in the greenhouse. Selected transformed lines were propagated vegetatively in the greenhouse either by tuber sowing or by explant cutting.
Plants were wounded or treated with a 50 μM JA solution as described (26). Systemic leaves were enclosed in polythene bags during spraying with JA and were uncovered after 30 minutes. Tuber yield was determined in plants, either nontransformed or a selected transgenic line, that were propagated in vitro and subsequently were transferred to soil in 500-ml pots, and were grown in the greenhouse. After 2 weeks, plants were transferred to 5-liter pots for tuber setting. Tubers were harvested simultaneously when senescing leaves appeared, usually after 3 months.
RNA and Protein Analysis.
Total RNA isolation and northern blotting techniques with 32P-radiolabeled cDNA probes were performed as described (26). For LOX-H3 RNA detection, noncoding-strand 32P-labeled riboprobes were prepared by using an in vitro transcription kit (Stratagene). Protein extraction and Western blot analysis were performed as described (31) by using the antibodies for potato proteinase inhibitor II (a kind gift from C. A. Ryan, Washington State University) or the antibodies for potato LOX-H1 and LOX-H3 (see below). A peroxidase-coupled goat anti-rabbit antibody was used to detect immunoreactive proteins using the enhanced chemiluminescence system (Amersham Pharmacia). Experiments were performed independently at least five times, yielding highly reproducible results. Single, representative experiments are shown in the figures.
Antibody Production.
The potato LOX-H1 protein produced in Escherichia coli (26) was used as immunogen to inject outbred New Zealand rabbits, and the sera obtained were used without further purification. To obtain antibodies recognizing the potato LOX-H3 protein, the peptides LGHLNGMTVQEALDA and TDMDPNTKGPKKSNQ, spanning amino acids 468–478 and 122–136 of its sequence, were synthesized (32) and coupled to keyhole limpet hemocyanin (Pierce) via N- and C-terminal cysteines, included for coupling purposes (33). These conjugates were used to immunize rabbits as described above. The antisera obtained specifically recognized (not shown) the LOX isoform used as immunogen when expressed in E. coli (26).
JA was coupled to keyhole limpet hemocyanin and was used to raise rabbit antibodies specific for jasmonates as described (34). Specificity was assessed by determining the relative cross-reactivity to JA–amino acid conjugates. Compared with methyl jasmonate (MeJA), +JA or −JA conjugated to l-Phe, l-Ile, or l-Val (a kind gift from C. Wasternack, Institut für Pflanzenbiochemie) showed a 25-fold lower reactivity. Some cross-reactivity was detected with +JA-d-Val (5× lower than MeJA) and with −JA-d-Val (3× lower). In this assay, ±JA, methyl salicylate, and abscisic acid and its methyl ester were below the limits of detection (at least 250× lower reactivity than MeJA).
Measurements of Jasmonate Concentration in Plant Tissue.
Three to four leaves were damaged per plant and were collected at the indicated times after wounding, as were leaves from nonwounded plants. After harvesting, leaves were frozen immediately in liquid nitrogen and were kept at −80°C until further process. From each sample, 0.5 g of leaf tissue were ground, and jasmonates were extracted overnight in 80% methanol, at 4°C with stirring. After centrifugation at 4°C, supernatants were cleared by passing through C-18 Sep-Pak cartridges (Waters). Eluates were vacuum-concentrated to aqueous phase, and 1/10 volume of 2 M sodium acetate pH 4.0 was added and extracted twice with 1 volume of dichloromethane. Organic phase was separated by low speed centrifugation, was transferred to a glass vial, and was vacuum-dried. Samples were methylated with an excess of freshly prepared diazomethane before analysis. A competitive ELISA assay was established by using multiwell, protein A-coated polystyrene plates to which jasmonate antibodies were fixed. Jasmonate concentrations in samples were determined by competition with JA coupled to alkaline phosphatase, using p-nitrophenylphosphate (Sigma) as reaction substrate. Quantitation of jasmonates in samples was done by using a calibration standard curve of MeJA (10–7,500 pmol). Values were corrected for the estimated recovery (55–85%) calculated by spiking samples with known amounts of JA and MeJA.
Insect–Plant Interactions.
Short-term feeding assays. Third instar Colorado potato beetle (CPB) larvae, reared on potato plants, were starved for 4 h. Three to four fully developed leaves were damaged per plant by confining one starved larva per leaf with a polythene bag (70 mm in diameter × 220 mm in length) clipped at the base of the petiole. Larvae were allowed to feed for 4 h on the leaves of the transgenic potato plants or on the nontransformed ones as control and then were removed. Thereafter, plants were allowed to build up their potential defenses for 16 h, and the leaves damaged previously were similarly reinfested with larvae of ≈40–45 mg each. Larval performance (as larval weight) on control and transgenic plants was determined 36 h after infestation. Six to eight plants per genotype were used; plants were held in a growth chamber at 24°C, 85% relative humidity, and 16:8 h light/dark photoperiod and were arranged in a completely randomized block design. For beet armyworm (BAW), fourth instar larvae reared on an artificial diet (35), weighing ≈40–44 mg each, were placed on the nontransformed and transgenic plants and were allowed to feed after the procedure described above.
Long-term feeding assays.
Procedure and environmental conditions were as in short-term assays, but second instar larvae (<24 h old, weighing 8–10 mg) were used and were allowed to feed for 4 days (until fourth larval instar).
RESULTS
Antisense-Mediated Depletion of a Specific 13-LOX Isoform Abolishes Wound-Induced Activation of Proteinase Inhibitor Genes.
13-LOX activity is a key step in the synthesis of the plant hormone JA (18, 19), which regulates gene activation in response to wounding (6, 7, 8). Two distinct 13-LOX genes (LOX-H1 and LOX-H3) expressed in wounded potato leaves had been identified (26). Our approach to elucidate their possible roles in the regulation of gene expression on wounding has been to generate transgenic potato lines in which these LOX isoforms are individually depleted by constitutive expression of their respective antisense RNAs.
As a first step toward this end, we have obtained 19 potato lines expressing an antisense LOX-H3 RNA from the 35S promoter. From them, two independent transformants (H3-3 and H3-4) showed greatly reduced levels of the cognate sense LOX-H3 mRNA. In fact, the transient increase in LOX-H3 transcript accumulation observed within 2 h after wounding in nontranformed plants is very much reduced in the H3-3 line and completely abolished in the H3-4 line (Fig. 1).
Figure 1.
Effects of antisense depletion of LOX-H3 mRNA on wound-induced gene expression. Leaves of nontransformed (WT) and LOX-H3 antisense lines H3-3 (#3) and H3-4 (#4) were wounded and harvested for total RNA extraction at the times indicated above the lanes (in hours). As control, leaves were collected from plants before wounding (c). Northern blots were hybridized with radioactive cDNA probes of the genes indicated on the left. For LOX-H3, an antisense-strand riboprobe was used to specifically detect LOX-H3 mRNA. RNA loading and transfer to the membrane was verified by hybridization with a 18S ribosomal DNA probe (rRNA). CDI, cathepsin D inhibitor.
In concert with this antisense-mediated inhibition of LOX-H3, a large reduction in the wound-induced accumulation of proteinase inhibitor transcripts was observed. In nontransformed plants, transcript levels of the proteinase inhibitor II (pin2) and cathepsin D inhibitor increased on wounding, with a time course clearly distinguishable from the one followed by the LOX-H3 gene. The levels of pin2 and cathepsin D inhibitor transcripts attained on wounding in both H3-3 and H3-4 transgenic lines were well below those observed in the nontransformed plants. Because this reduction was seen clearly in two independent transgenic lines, it is most likely related to the depletion of LOX-H3 engineered in these plants. In addition, the transcript accumulation of other wound-inducible genes, the LOX-H1 for instance, is also affected, albeit to a lower extent in the LOX-H3 antisense plants. Both the antisense-mediated depletion of LOX-H3 transcripts and the concerted reduction in the accumulation of wound-inducible transcripts is maintained when the transgenic lines are vegetatively propagated, either by explant replication in vitro or in soil by tuber sowing (not shown).
CPBs and BAWs Fed on LOX-H3-Depleted Plants Have Higher Weight Gain Rates.
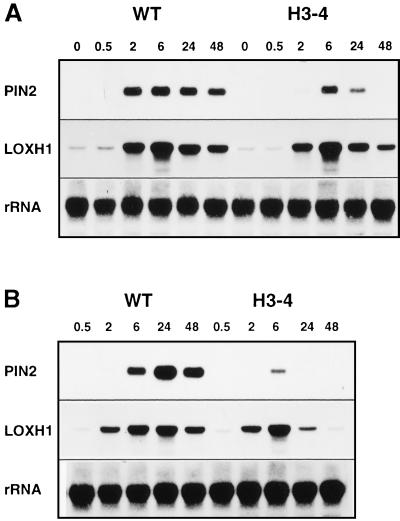
On wounding, potato plants devoid of LOX-H3 accumulate very low levels of pin2 and cathepsin D inhibitor transcripts, which are thought to be involved in plant resistance to pest attack. Insect feeding had little effect on LOX-H3 mRNA accumulation at the times analyzed (Fig. 2A), which, however, do not include the peak times for LOX-H3 mRNA accumulation on mechanical wounding (see Fig. 1). In nontransformed plants, a weak band immunoreactive with both LOX-H3-specific antibodies was detected at the latest time point analyzed (Fig. 2B). Accumulation of this protein is very much reduced in the H3-4 plants (Fig. 2B), indicating that the protein detected is a bona fide LOX-H3. The significant divergence in patterns of LOX-H3 protein and mRNA accumulation (Fig. 2 A and B) suggests that post-transcriptional mechanisms are involved in the regulation of LOX-H3. This also seems to be the case for LOX-H1. Similar to wounding, insect feeding causes accumulation of LOX-H1 mRNA to higher levels in the nontranformed plants than in the antisense ones, in which little alterations are detected in response to insect feeding. However, LOX-H1 protein levels are nearly identical in nontransformed and H3-4 plants and remain unaffected by the chewing of insects.
Figure 2.
Effects of insect feeding on the accumulation of wound-inducible gene products. Leaves of nontransformed (wt) potatoes and plants from a LOX-H3 antisense line (H3-4) were collected before (0) or at the indicated times (in hours) after long-term infestation with CPB larvae. Total RNA (A) and protein (B) were isolated, and LOX-H1, LOX-H3, and pin2 products were identified by hybridization to the corresponding cDNA probes (Northern blots in A) and antisera (Western blots in B). Equal RNA loading on Northern blots was verified by hybridization with a 18S ribosomal cDNA probe (rRNA). Equal protein loading was verified by visualization of the large subunit of Rubisco (RBCL) stained with Ponceau Red.
Larvae of both insect species feeding in nontransformed plants induce the accumulation of pin2 mRNA and protein in the leaf on which they are feeding (Fig. 2 and data not shown). Very little pin2 accumulation is detected in the leaves of the same plant that are not being eaten by the insects within the time span analyzed (not shown). In contrast, feeding of larvae on H3-4 plants did not result in detectable pin2 accumulation, either in the leaves that were eaten directly or in the systemic, nondamaged ones.
Larvae of CPB and BAW were fed ad libitum on potato leaves from either control or H3-4 plants to assess the effects of the presence of proteinase inhibitors and other defensive compounds on larval growth. Larvae of CPB fed on H3-4 plants from second to fourth instar (long-term feeding assays) had significantly higher weights (30%) than those fed on nontransformed plants (Table 1). When third instar CPB larvae were fed for 36 h (short-term feeding assays) on H3-4 plants, a 12% higher weight gain over larvae fed in control plants was observed. The differences in weight gain were even larger for BAWs, with a 57% higher weight of fourth instar larvae fed on H3-4 plants for 48 h over those fed in nontransformed ones (Table 1).
Table 1.
Performance of CPB and BAW larvae fed on nontransformed (control) potatoes and the LOX-H3-depleted H3-4 line
| Feeding assay† | Treatment | Larval fresh weight,‡ mg
|
||
|---|---|---|---|---|
| Initial | Final | Weight gain | ||
| Short-term (CPB) | Control | 41.6 ± 0.5 | 96.7 ± 1.3 | 55.1 ± 1.4 |
| H3-4 | 42.3 ± 0.4 | 103.5 ± 1.4* | 61.5 ± 1.4* | |
| Long-term (CPB) | Control | 9.0 ± 0.1 | 83.9 ± 4.6 | 74.9 ± 4.6 |
| H3-4 | 9.1 ± 0.1 | 106.5 ± 5.9* | 97.5 ± 5.9* | |
| Short-term (BAW) | Control | 42.3 ± 0.2 | 113.1 ± 3.2 | 70.8 ± 3.1 |
| H3-4 | 42.2 ± 0.2 | 153.1 ± 4.3 | 111.0 ± 4.2* | |
Asterisk denotes significant differences with control plants at P < 0.05 (Newman-Keuls test) in each feeding assay.
Short-term (CPB), third instar larvae fed for 36 h; long-term (CPB), second instar larvae fed for 4 days; short-term (BAW), fourth instar larvae fed for 48 h.
Larval weight values are mean ± SD (n = 24).
The Reduced Accumulation of Wound Inducible Genes in LOX-H3-Depleted Plants Is Not Mediated by Large Alterations in the JA Levels Attained After Wounding.
A 13-LOX activity has been shown to be required for the wound-induced synthesis of JA and hence for the activation of wound inducible genes (20). It could thus be expected that the effect of the depletion of LOX-H3 on the wound-induced activation of defensive genes was attributable to its involvement in JA synthesis. However, treatment of the antisense H3-3 and H3-4 plants with JA did not reverse that effect. Indeed, JA treatment of nontransformed plants led to the accumulation of pin2 transcripts in both the directly sprayed leaves (Fig. 3A) as well as in systemic, nonsprayed ones (Fig. 3B). In contrast, the accumulation of pin2 transcripts after JA treatment of H3-4 plants was very low in the local leaves (Fig. 3A) and largely was abolished in the systemic ones (Fig. 3B). In contrast, LOX-H1 mRNA accumulates to nearly wild-type levels in H3-4 plants after JA treatment, in particular in the sprayed leaves (Fig. 3A). These data suggest that exogenous JA is being perceived both in the nontransformed plants and in the antisense ones but, however, is not able to alleviate the effect of LOX-H3 depletion on the induction of proteinase inhibitors.
Figure 3.
Complementation of the effects of LOX-H3 depletion by exogenous treatment with jasmonic acid. (A) Leaves of nontransformed (WT) and LOX-H3-depleted (H3-4) plants sprayed with a 50 μM JA solution were harvested for total RNA extraction at the times after treatment indicated (in hours) above the lanes. Control leaves were collected before JA treatment (0). (B) Some leaves from the plants used for the experiments shown in A were enclosed in polythene bags before treatment to prevent direct spraying with JA. These systemic leaves were collected at the same time as the locally treated ones, as indicated, and were used for total RNA isolation. Northern blots were hybridized with radioactive cDNA probes as indicated on the left. Equal RNA loading was verified by hybridization with a 18S ribosomal cDNA probe (rRNA).
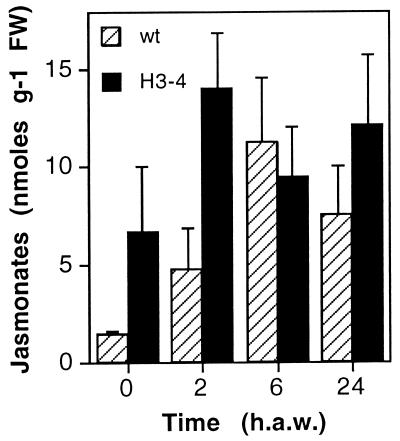
The endogenous jasmonate levels were determined in H3-3 and H3-4 plants both before and at different times after wounding. The data showed in Fig. 4 indicate that jasmonate levels in wounded H3-4 plants reached similar levels to those determined in nontransformed ones. Moreover, jasmonate concentrations in nonwounded leaves or in the leaves at early times after wounding in the antisense lines are slightly higher than in nontransformed plants. H3-3 plants had intermediate levels of jasmonates (not shown). Taken together, these data indicate that LOX-H3 is not involved in the synthesis of JA, which leads to the increase in endogenous levels observed in wounded leaves.
Figure 4.
Jasmonate levels in nontransformed and LOX-H3-depleted plants. Leaves from nontransformed (WT) or antisense (H3-4) plants were harvested before (0) or at the indicated times after being wounded [2, 6, and 24 h after wounding (h.a.w.)], and their jasmonate content was determined by a competitive ELISA assay. Data are given in nanomoles of jasmonate per gram of fresh weight of leaves, and represented is a single experiment with the average ± SD (bars) concentration in six plants each for WT and H3-4, each single time point determined in triplicate from different tissue samples. The experiments were repeated two more times, yielding highly reproducible results.
LOX-H3 Depleted Plants Have a Higher Tuber Yield.
It has been reported that JA may act as a tuber-inducing factor (27). It was, therefore, of interest to determine whether an alteration in an enzyme activity that may participate in JA biosynthesis could influence tuber formation. To this end, tuber yield was determined for both nontransformed and H3-3 and H3-4 plants. From the results obtained it can be seen that depletion of LOX-H3 reproducibly leads to higher tuber yields. H3-4 plants grown in the greenhouse yield, on average, 20% more tuber weight per plant than do nontransformed ones (Table 2) whereas H3-3 have intermediate values (not shown). Water accounts for 75–80% of the tuber weight in both nontransformed and LOX-H3-depleted plants, and the number of tubers produced by both are not significantly different (not shown). The higher tuber yield observed in both antisense lines together with the correlation of tuber weight per plant and the residual levels of LOX-H3 are again indicative of a direct relation between LOX-H3 depletion and tuber yield. Tuber yield is also consistent with the higher basal jasmonate content of the transgenic lines.
Table 2.
Tuber yields of nontransformed potatoes and the LOX-H3 depleted H3-4 line
| Month | n | WT (tfw) | H3-4 (tfw) | Percent over WT |
|---|---|---|---|---|
| August | 9 | 140.8 ± 51.5 | 182.6 ± 33.2 | 30 |
| September | 7 | 223.3 ± 22.5 | 249.6 ± 32.2 | 12 |
| October | 5 | 190.4 ± 20.4 | 235.1 ± 16.6 | 23 |
| April | 8 | 160.5 ± 47.8 | 185.5 ± 14.1 | 16 |
Data presented indicate the month the tubers were harvested and the number (n) of nontransformed (WT) and LOX-H3-depleted (H3-4) plants used for each independent experiment. Yields are given in grams as average tuber fresh weight (tfw) per plant ±SD. The increase in total tuber weight in H3-4 plants over the nontransformed ones (arbitrarily taken as 100%) is given (Percent over WT).
DISCUSSION
The antisense-mediated depletion of LOX-H3 in transgenic potato plants leads to large reductions in the wound-induced accumulation of proteinase inhibitors. Because this effect has been observed in two transgenic lines independently obtained and, moreover, the extent of reduction in proteinase inhibitor levels correlates well with the residual LOX-H3 present in the transgenic lines, it can be ascribed confidently to the depletion of LOX-H3 engineered in these lines. LOX-H3 appears, thus, to be a master gene in the regulation of coordinated gene activation in response to wounding.
Inducible defense systems play an instrumental role in plant–pest interactions (11, 15). Consistent with this assumption, larvae of CPB (preconditioned on potato plants) fed on LOX-H3-depleted plants gained 12–30% more weight than larvae fed for the same period of time in nontransformed plants. The effect of LOX-H3 depletion on insect performance should, however, be more apparent in BAW as a generalist herbivore than in the specialized CPB, more adapted to the inducible chemical weaponry of potato. Indeed, as much as 57% larger weight gains could be determined when BAW, previously reared on artificial diet, subsequently was fed on these antisense plants over those fed on nontransformed ones. The 5-fold gain of weight of a generalist versus a specialized potato leaf-feeding insect in short-term assays suggests a more efficient dealing with host plant defenses of the latter, likely because of its ability to overcome the action of plant proteinase inhibitors (36). Taken together, these data suggest that the accumulation of proteinase inhibitors induced in potato by insect chewing is relevant for prevention of pest attack, in particular for generalist leaf-feeding herbivores. Because LOX-H3 depletion abates protection against two different insect pests, LOX-H3 appears to be a key regulatory gene in the defensive response of potato toward pest attack.
It has to be kept in mind, however, that LOX-H3 depletion not only affects the accumulation of proteinase inhibitors after wounding but also the concerted induction of LOX-H1 and other genes that also may serve defensive functions (37). It is thus likely that resistance to pest attack is the result of the coordinated accumulation of secondary metabolites and protein products, some of which may directly interfere with digestibility of the ingested tissue as the proteinase inhibitors whereas others may affect food intake (38).
In any case, LOX-H3 appears to be instrumental for wound-induced gene activation. There is, however, no evidence to support that the involvement of LOX-H3 in gene induction on wounding is attributable to its enzymatic activity and the production of putative precursors in the JA biosynthetic pathway. Indeed, jasmonate concentration attains similar levels after wounding wild-type and LOX-H3-depleted lines, suggesting that LOX-H3 activity is not required for the synthesis of the bulk of wound-induced jasmonates. It previously was shown that, in Arabidopsis, separate biosynthetic pathways could be responsible for wound-induced and basal JA levels (20). In the case of potato, LOX-H3 depletion does not result in a reduction of basal jasmonate levels either. They appear, if at all, to be higher in the antisense lines than in nontransformed plants, suggesting that LOX-H3 may play a regulatory role over other activities, perhaps a different LOX isoform, involved in JA biosynthesis. This possibility may be relevant regarding LOX-H1 expression. LOX-H1 mRNA accumulation in response to pest attack was very much reduced in the LOX-H3 depleted plants, but LOX-H1 protein levels largely were unaffected, suggesting a low rate of LOX-H1 protein turnover. However, antisense depletion of LOX-H1 mRNA results in the concomitant reduction of LOX-H1 protein (not shown), suggesting that the presence of LOX-H3 in these plants may negatively affect translatability of the remaining LOX-H1 mRNA, perhaps via JA (39), or may interfere directly with LOX-H1 protein stability.
Exogenous application of JA is not able to recover wild-type proteinase inhibitor levels in the LOX-H3 antisense plants, in agreement with the normal endogenous jasmonate content of these plants. Nontransformed plants accumulate proteinase inhibitor and LOX-H1 transcripts to similar levels in both the locally JA-treated leaves and the nontreated, systemic ones. In contrast, LOX-H3-depleted plants accumulate much reduced amounts of proteinase inhibitor transcripts on JA treatment, in particular in the systemic leaves. However, LOX-H1 mRNA attains nearly wild-type levels in the JA treated H3-4 plants. LOX-H3 thus appears to be required for production of a signal that cannot be mimicked by exogenous JA application and that regulates pin2 expression. It has been reported that, in tomato plants, ethylene is required, together with JA, for maximal pin2 induction (40). It is tempting to speculate that LOX-H3, or its products, regulates ethylene action on wound-induced expression of proteinase inhibitors. LOX-H3 may participate in this regulatory network by providing minute amounts of JA, constituting a distinct pool, nondetectable against the high wound-induced JA levels but playing, nonetheless, a pivotal role in the activation of the defense response. Alternatively, the fatty acid hydroperoxide products of LOX-H3 may be required for the enzymatic activation (23) of a different LOX isoform.
LOX-H3-depleted plants are largely indistinguishable from nontransformed ones. However, a characteristic feature of LOX-H3 depletion is the higher tuber yield of the antisense lines, on average 20% more tuber weight per plant than the nontransformed ones. This may be because of the higher basal jasmonate levels present in the transformed lines, and thus consistent with the suggested role of JA in promoting tuberization (27). An alternative explanation, however, could invoke a better performance in general fitness of the transformed plants as a side effect of inactivating this inducible defense system and would be reflected not only in tuber weight but also in other parameters such as higher biomass or flower setting. LOX-H3-antisense plants appear indeed to set more flowers than nontransformed ones (not shown), lending support to the intriguing possibility that the maintenance of an inducible defense system may pose a penalty in fitness in nonchallenged plants, likely to be outweighed by a better performance on pest challenge. This hypothesis is consistent with the recent data on the cost of jasmonate-induced responses in fitness of Nicotiana plants (41). LOX-H3 thus appears to be a main target for engineering plants for resistance to pest attack. Understanding the role of LOX-H3 in the regulation of gene activation on wounding is required for preventing undesired side effects resulting from the pleiotropic effects of LOX activity in plants.
Acknowledgments
We thank Prof. C. A. Ryan for potato pin2 antibodies and Prof. C. Wasternack for the JA conjugates. We are grateful to Alfonso Valencia and Luis Sánchez Pulido for computer predictions of LOX-H3 and help in designing antigenic peptides. Our gratitude goes to Rod Casey for helpful suggestions and comments on the manuscript. Tomás Cascón provided excellent technical assistance. We also gratefully acknowledge the photographic work by Inés Poveda and Angel Sanz. Financial support was provided by the Spanish Comision Interministerial de Ciencia y Tecnologia Grant BIO96-0532-CO2-01 to J.J.S.-S. and AGF98-0805-CO2-01 to P.C. The Departamento de Inmunología y Oncología Centro Nacional de Biotechnología is supported by the Spanish Consejo Superior de Investigaciones Científicas and Pharmacia-Upjohn. J.R. and J.L. were supported by postdoctoral contracts, and G.V. was supported by a postdoctoral fellowship from the Spanish Ministerio de Educación y Ciencia.
ABBREVIATIONS
- BAW
beet armyworm
- CPB
Colorado potato beetle
- JA
jasmonic acid
- MeJA
methyl jasmonate
- LOX
lipoxygenase
- pin2
proteinase inhibitor II
References
- 1.Agrawal A. Science. 1998;279:1201–1202. doi: 10.1126/science.279.5354.1201. [DOI] [PubMed] [Google Scholar]
- 2.Bernays E A, Chapman R F. Host–Plant Selection by Phytophagous Insects. New York: Chapman & Hall; 1994. p. 312. [Google Scholar]
- 3.Bowles D J. Annu Rev Biochem. 1990;59:873–907. doi: 10.1146/annurev.bi.59.070190.004301. [DOI] [PubMed] [Google Scholar]
- 4.Alborn H T, Turlings T C J, Jones T H, Stenhagen G, Loughrin J H, Tumlinson J H. Science. 1997;276:945–949. [Google Scholar]
- 5.Ryan C A. Annu Rev Phytopathol. 1990;28:425–449. [Google Scholar]
- 6.Farmer E E, Ryan C A. Plant Cell. 1992;4:129–134. doi: 10.1105/tpc.4.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Creelman R A, Mullet J E. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:355–381. doi: 10.1146/annurev.arplant.48.1.355. [DOI] [PubMed] [Google Scholar]
- 8.Wasternack C, Parthier B. Trends Plant Sci. 1997;2:302–307. [Google Scholar]
- 9.Peña-Cortés H, Fisahn J, Willmitzer L. Proc Natl Acad Sci USA. 1995;92:4106–4113. doi: 10.1073/pnas.92.10.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doares S H, Syrovets T, Weiler E W, Ryan C A. Proc Natl Acad Sci USA. 1995;92:4095–4098. doi: 10.1073/pnas.92.10.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howe G A, Lightner J, Browse J, Ryan C A. Plant Cell. 1996;8:2067–2077. doi: 10.1105/tpc.8.11.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peña-Cortés H, Albrecht T, Prat S, Weiler E E, Willmitzer L. Planta. 1993;191:123–128. [Google Scholar]
- 13.McGurl B, Pearce G, Orozco-Cárdenas M L, Ryan C A. Science. 1992;255:1570–1573. doi: 10.1126/science.1549783. [DOI] [PubMed] [Google Scholar]
- 14.Peña-Cortés H, Willmitzer L, Sánchez-Serrano J J. Plant Cell. 1991;3:963–972. doi: 10.1105/tpc.3.9.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orozco-Cárdenas M, McGurl B, Ryan C A. Proc Natl Acad Sci USA. 1993;90:8273–8276. doi: 10.1073/pnas.90.17.8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McConn M, Creelman R A, Bell E, Mullet J E, Browse J. Proc Natl Acad Sci USA. 1997;94:5473–5477. doi: 10.1073/pnas.94.10.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conconi A, Miquel M, Browse J A, Ryan C A. Plant Physiol. 1996;111:797–803. doi: 10.1104/pp.111.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosahl S. Z Naturforsch, C. 1996;51:123–138. doi: 10.1515/znc-1996-3-401. [DOI] [PubMed] [Google Scholar]
- 19.Vick B A. In: Lipid Metabolism in Plants. Moore T S, editor. Boca Raton, FL: CRC; 1993. pp. 167–191. [Google Scholar]
- 20.Bell E, Creelman R A, Mullet J E. Proc Natl Acad Sci USA. 1995;92:8675–8679. doi: 10.1073/pnas.92.19.8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gundlach H, Zenk M H. Phytochemistry. 1998;47:527–537. doi: 10.1016/s0031-9422(98)00275-1. [DOI] [PubMed] [Google Scholar]
- 22.Weber H, Vick B A, Farmer E E. Proc Natl Acad Sci USA. 1997;94:10473–10478. doi: 10.1073/pnas.94.19.10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siedow J N. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:145–188. [Google Scholar]
- 24.Rancé I, Fournier J, Esquerré-Tugayé M T. Proc Natl Acad Sci USA. 1998;95:6554–6559. doi: 10.1073/pnas.95.11.6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geerts A, Feltkamp D, Rosahl S. Plant Physiol. 1994;105:269–277. doi: 10.1104/pp.105.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Royo J, Vancanneyt G, Pérez A G, Sanz C, Stoermann K, Rosahl S, Sánchez-Serrano J J. J Biol Chem. 1996;271:21012–21019. doi: 10.1074/jbc.271.35.21012. [DOI] [PubMed] [Google Scholar]
- 27.Koda Y, Kikuta Y, Tazaki H, Tsujino Y, Sakamura S, Yoshihara T. Phytochemistry. 1991;30:1435–1438. [Google Scholar]
- 28.Keil M, Sánchez-Serrano J J, Willmitzer L. EMBO J. 1989;8:1323–1330. doi: 10.1002/j.1460-2075.1989.tb03512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bevan M. Nucleic Acids Res. 1984;12:8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Höfgen R, Willmitzer L. Plant Sci. 1990;66:221–230. [Google Scholar]
- 31.Dammann C, Rojo E, Sánchez-Serrano J J. Plant J. 1997;11:773–782. doi: 10.1046/j.1365-313x.1997.11040773.x. [DOI] [PubMed] [Google Scholar]
- 32.Gausepohl H, Boulin C, Kraft M, Frank R W. Peptide Res. 1992;5:315–320. [PubMed] [Google Scholar]
- 33.Hashida S, Imagawa M, Inque S, Ruan K H, Ishikawa E. J Appl Biochem. 1984;6:56–63. [PubMed] [Google Scholar]
- 34.Knöfel H D, Brückner C, Kramell R, Sembdner G, Schreiber K. Biochem Physiol Pflanz. 1990;186:387–394. [Google Scholar]
- 35.Poitout S, Bues R. Ann Zool Ecol Anim. 1970;2:79–91. [Google Scholar]
- 36.Jongsma M A, Bakker P L, Peters J, Bosch D, Stiekema W J. Proc Natl Acad Sci USA. 1995;92:8041–8045. doi: 10.1073/pnas.92.17.8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hildmann T, Ebneth M, Peña-Cortés H, Sánchez-Serrano J J, Willmitzer L, Prat S. Plant Cell. 1992;4:1157–1170. doi: 10.1105/tpc.4.9.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duffey S S, Stout M J. Arch Insect Biochem Physiol. 1996;32:3–37. [Google Scholar]
- 39.Reinbothe S, Reinbothe C, Parthier B. J Biol Chem. 1993;268:10606–10611. [PubMed] [Google Scholar]
- 40.O’Donnell P J, Calvert C, Atzorn R, Wasternack C, Leyser H M O, Bowles D J. Science. 1996;274:1914–1917. doi: 10.1126/science.274.5294.1914. [DOI] [PubMed] [Google Scholar]
- 41.Baldwin I T. Proc Natl Acad Sci USA. 1998;95:8113–8118. doi: 10.1073/pnas.95.14.8113. [DOI] [PMC free article] [PubMed] [Google Scholar]