Abstract
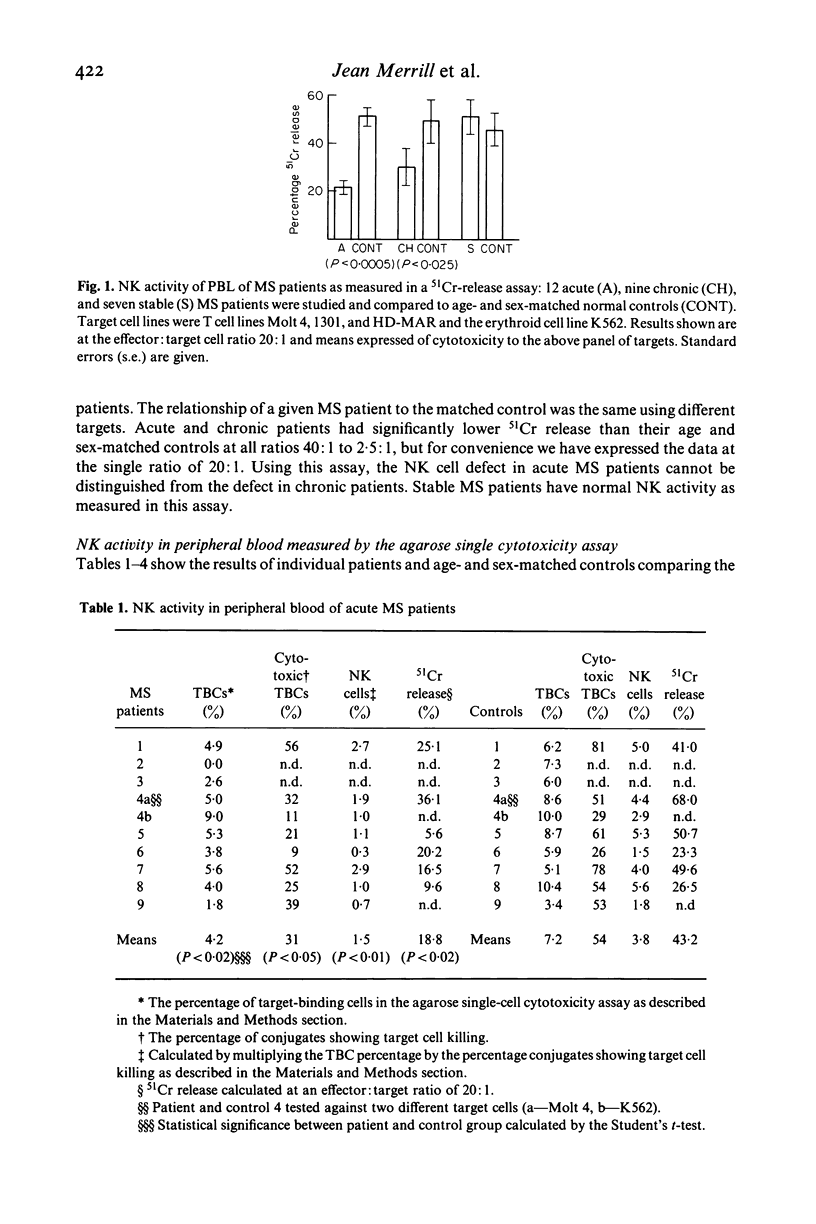
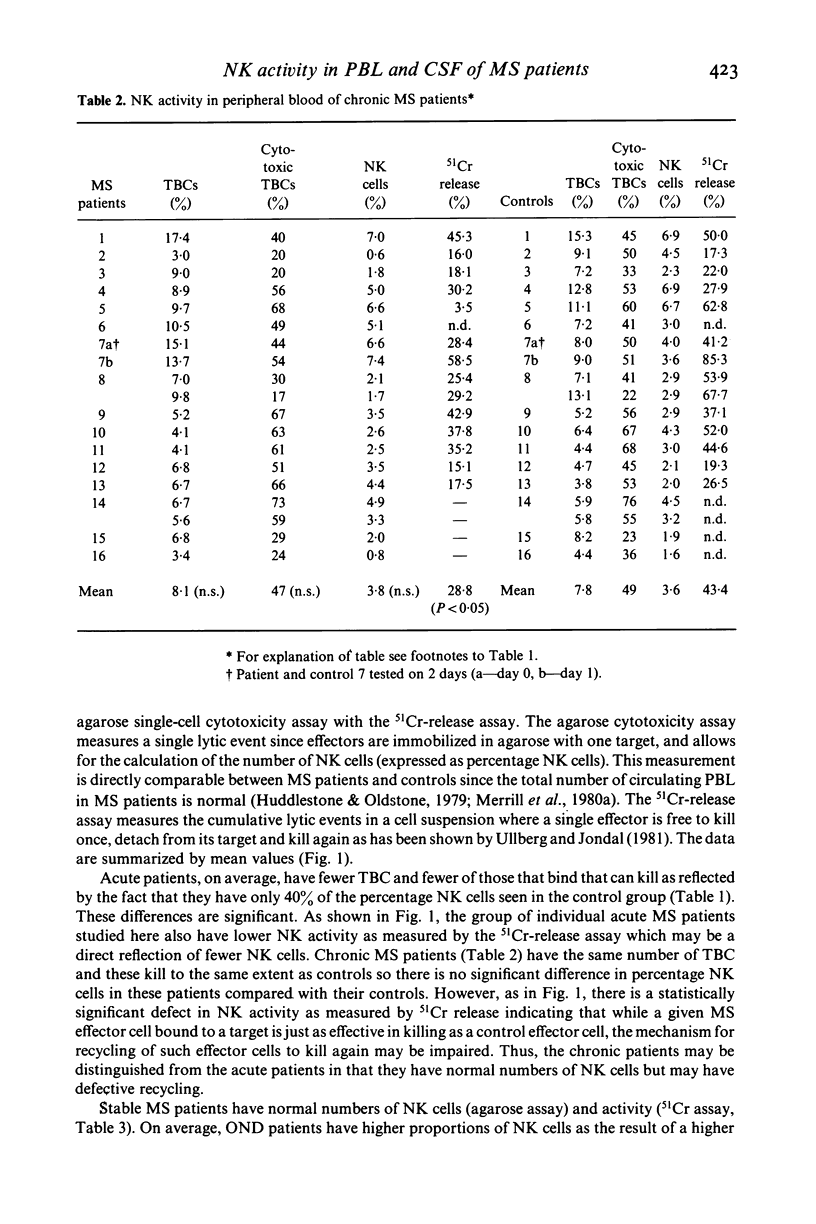
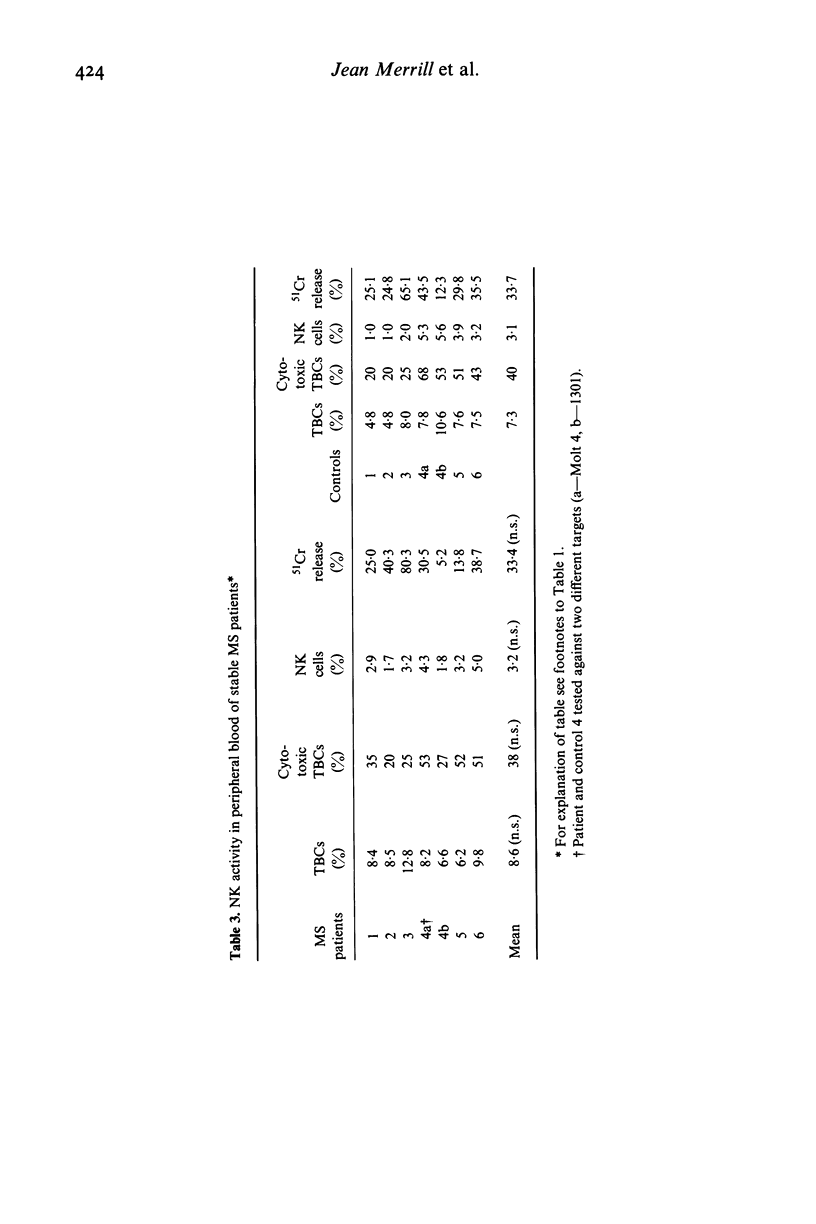
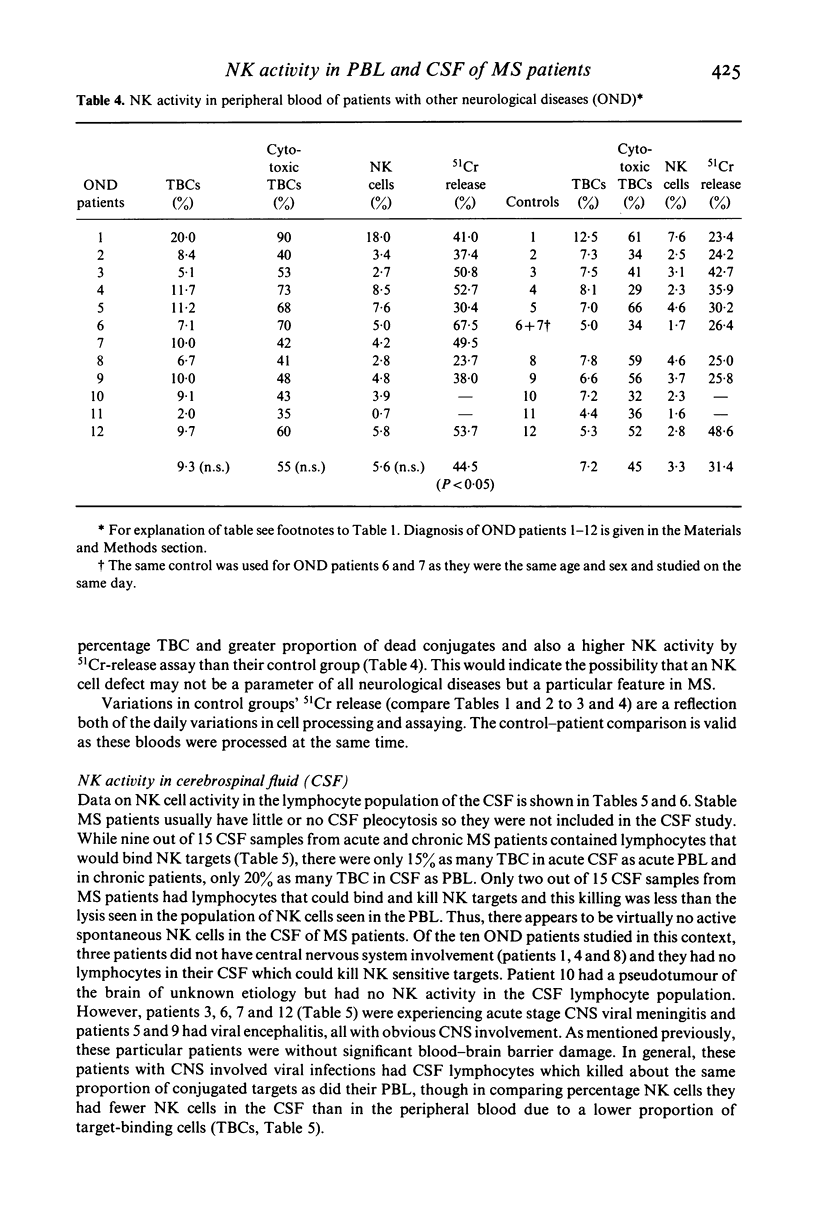
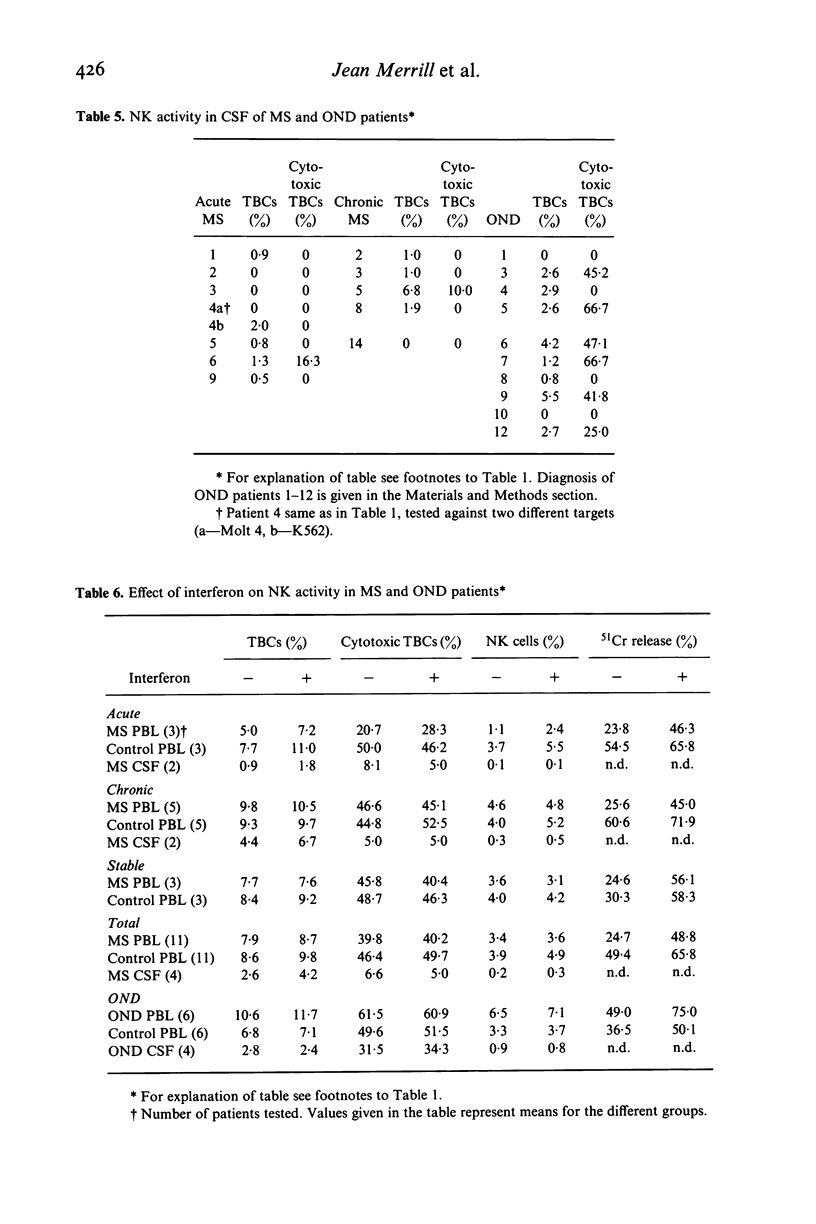
Natural killer cell activity has earlier been shown to be depressed in patients with multiple sclerosis (MS) (Benczur et al., 1980). In the present study, this defect was more clearly characterized in different stages of the disease. By using a single-cell cytotoxicity assay in agarose (Grimm & Bonavida, 1979), in combination with the conventional 51Cr-release, the number of target-binding cells (TBCs) and the fraction of active killer cells therein could be compared with the radioisotope release in the different patient groups. It was found that patients with active and chronic MS showed lower natural killer (NK) activity in the 51Cr-release assay as compared with age and sex-matched controls, in contrast to stable MS patients who were comparable with their control group. The single cell cytotoxicity assay demonstrated that acute MS patients had a decreased number of TBCs in peripheral blood and that they also had a decreased percentage of active NK cells in their TBC fractions. Patients with chronic MS were normal in the single-cell cytotoxicity assay. When cells present in CSF were analysed in acute and chronic MS, few cells were found with target binding capacity and only in two instances out of 13 could any cytotoxicity at all be detected. Patients with other neurological diseases (OND) were found to have detectable NK activity in CSF in six cases out of ten in the single-cell assay. OND patients as a group also had higher peripheral NK activity in the 51Cr-release assay as compared with the control group. When peripheral and CSF cells from MS patients and OND patients were treated with interferon, no increase in TBCs or fraction of killer cells in TBCs was found. In the 51Cr-release assay, comparable increases in cytotoxicity were found in all groups. One possible explanation for the stage-related NK suppression seen in the present investigation may be a decreased interferon production combined with immune-complex induced, macrophage-produced prostaglandins which are known to influence the human NK system in a comparable way in vitro. Additionally, immune-complexes may directly interact with human NK cells as the majority of these are known to express receptors for the Fc portion of IgG.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnason B. G., Antel J. Suppressor cell function in multiple sclerosis. Ann Immunol (Paris) 1978 Feb-Mar;129(2-3):159–170. [PubMed] [Google Scholar]
- Benczur M., Petrányl G. G., Pálffy G., Varga M., Tálas M., Kotsy B., Földes I., Hollán S. R. Dysfunction of natural killer cells in multiple sclerosis: a possible pathogenetic factor. Clin Exp Immunol. 1980 Mar;39(3):657–662. [PMC free article] [PubMed] [Google Scholar]
- Brunda M. J., Herberman R. B., Holden H. T. Inhibition of murine natural killer cell activity by prostaglandins. J Immunol. 1980 Jun;124(6):2682–2687. [PubMed] [Google Scholar]
- Bøyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol. 1976 Jun;Suppl 5:9–15. [PubMed] [Google Scholar]
- Davison A. N. Progress in recent research on multiple sclerosis. Biochem Soc Trans. 1978;6(2):443–447. doi: 10.1042/bst0060443. [DOI] [PubMed] [Google Scholar]
- Droller M. J., Schneider M. U., Perlmann P. A possible role of prostaglandins in the inhibition of natural and antibody-dependent cell-mediated cytotoxicity against tumor cells. Cell Immunol. 1978 Aug;39(1):165–177. doi: 10.1016/0008-8749(78)90091-6. [DOI] [PubMed] [Google Scholar]
- Einhorn S., Blomgren H., Strander H. Interferon and spontaneous cytotoxicity in man. V. Enhancement of spontaneous cytotoxicity in patients receiving human leukocyte interferon. Int J Cancer. 1980 Oct 15;26(4):419–428. doi: 10.1002/ijc.2910260406. [DOI] [PubMed] [Google Scholar]
- Gonzalez R. L., Dau P. C., Spitler L. E. Altered regulation of mitogen responsiveness by suppressor cells in multiple sclerosis. Clin Exp Immunol. 1979 Apr;36(1):78–84. [PMC free article] [PubMed] [Google Scholar]
- Goust J. M., Chenais F., Carnes J. E., Hames C. G., Fudenberg H. H., Hogan E. L. Abnormal T cell subpopulations and circulating immune complexes in the Guillain-Barré syndrome and multiple sclerosis. Neurology. 1978 May;28(5):421–425. doi: 10.1212/wnl.28.5.421. [DOI] [PubMed] [Google Scholar]
- Grimm E., Bonavida B. Mechanism of cell-mediated cytotoxicity at the single cell level. I. Estimation of cytotoxic T lymphocyte frequency and relative lytic efficiency. J Immunol. 1979 Dec;123(6):2861–2869. [PubMed] [Google Scholar]
- Huddlestone J. R., Oldstone M. B. T suppressor (TG) lymphocytes fluctuate in parallel with changes in the clinical course of patients with multiple sclerosis. J Immunol. 1979 Oct;123(4):1615–1618. [PubMed] [Google Scholar]
- Jondal M., Pross H. Surface markers on human b and t lymphocytes. VI. Cytotoxicity against cell lines as a functional marker for lymphocyte subpopulations. Int J Cancer. 1975 Apr 15;15(4):596–605. doi: 10.1002/ijc.2910150409. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Levitt D., Griffin N. B., Egan M. L. Mitogen-induced plasma cell differentiation in patients with multiple sclerosis. J Immunol. 1980 May;124(5):2117–2121. [PubMed] [Google Scholar]
- Levy N. L., Schoen T. M. Spinal fluid from patients with multiple sclerosis contains nucleotide-rich material associated with IgG. Neurology. 1976 Jun;26(6 Pt 2):62–63. doi: 10.1212/wnl.26.6_part_2.62. [DOI] [PubMed] [Google Scholar]
- Merrill J. E., Biberfeld G., Kolmodin G., Landin S., Norrby E. A T lymphocyte subpopulation in multiple sclerosis patients bearing Fc receptors for both IgG and IgM1. J Immunol. 1980 Jun;124(6):2758–2764. [PubMed] [Google Scholar]
- Merrill J. E., Biberfeld G., Landin S., Sidén A., Norrby E. Identification of three FcR-positive T cell subsets (T gamma, T mu and T gamma mu) in the cerebrospinal fluid of multiple sclerosis patients. Clin Exp Immunol. 1980 Nov;42(2):345–354. [PMC free article] [PubMed] [Google Scholar]
- Neighbour P. A., Bloom B. R. Absence of virus-induced lymphocyte suppression and interferon production in multiple sclerosis. Proc Natl Acad Sci U S A. 1979 Jan;76(1):476–480. doi: 10.1073/pnas.76.1.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker L. J., Raff H. V., Goldyne M. E., Stobo J. D. Metabolic heterogeneity among human monocytes and its modulation by PGE2. J Immunol. 1980 Jun;124(6):2557–2562. [PubMed] [Google Scholar]
- Reinherz E. L., Weiner H. L., Hauser S. L., Cohen J. A., Distaso J. A., Schlossman S. F. Loss of suppressor T cells in active multiple sclerosis. Analysis with monoclonal antibodies. N Engl J Med. 1980 Jul 17;303(3):125–129. doi: 10.1056/NEJM198007173030303. [DOI] [PubMed] [Google Scholar]
- SCHUMACHER G. A., BEEBE G., KIBLER R. F., KURLAND L. T., KURTZKE J. F., MCDOWELL F., NAGLER B., SIBLEY W. A., TOURTELLOTTE W. W., WILLMON T. L. PROBLEMS OF EXPERIMENTAL TRIALS OF THERAPY IN MULTIPLE SCLEROSIS: REPORT BY THE PANEL ON THE EVALUATION OF EXPERIMENTAL TRIALS OF THERAPY IN MULTIPLE SCLEROSIS. Ann N Y Acad Sci. 1965 Mar 31;122:552–568. doi: 10.1111/j.1749-6632.1965.tb20235.x. [DOI] [PubMed] [Google Scholar]
- Santoli D., Koprowski H. Mechanisms of activation of human natural killer cells against tumor and virus-infected cells. Immunol Rev. 1979;44:125–163. doi: 10.1111/j.1600-065x.1979.tb00269.x. [DOI] [PubMed] [Google Scholar]
- Santoli D., Moretta L., Lisak R., Gilden D., Koprowski H. Imbalances in T cell subpopulations in multiple sclerosis patients. J Immunol. 1978 Apr;120(4):1369–1371. [PubMed] [Google Scholar]
- Schliep G., Felgenhauer K. Serum-CSF protein gradients, the blood-GSF barrier and the local immune response. J Neurol. 1978 May 18;218(2):77–96. doi: 10.1007/BF02402169. [DOI] [PubMed] [Google Scholar]
- Sindic C. J., Cambiaso C. L., Masson P. L., Laterre E. C. The binding of myelin basic protein to the Fc region of aggregated IgG and to immune complexes. Clin Exp Immunol. 1980 Jul;41(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Sindic C. J., Cambiaso C. L., Masson P. L., Laterre E. C. The elution of IgG from subacute sclerosing panencephalitis and multiple sclerosis brains. Clin Exp Immunol. 1980 Jul;41(1):8–12. [PMC free article] [PubMed] [Google Scholar]
- Timonen T., Saksela E. Human natural cell-mediated cytotoxicity against fetal fibroblasts. I. General characteristics of the cytotoxic activity. Cell Immunol. 1977 Oct;33(2):340–352. doi: 10.1016/0008-8749(77)90163-0. [DOI] [PubMed] [Google Scholar]
- Trinchieri G., Santoli D. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Enhancement of human natural killer cell activity by interferon and antagonistic inhibition of susceptibility of target cells to lysis. J Exp Med. 1978 May 1;147(5):1314–1333. doi: 10.1084/jem.147.5.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullberg M., Jondal M. Recycling and target binding capacity of human natural killer cells. J Exp Med. 1981 Mar 1;153(3):615–628. doi: 10.1084/jem.153.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M., Jr, Zinkernagel R. M. Heterospecific cytotoxic cell activity induced during the first three days of acute lymphocytic choriomeningitis virus infection in mice. Nature. 1977 Aug 18;268(5621):646–648. doi: 10.1038/268646a0. [DOI] [PubMed] [Google Scholar]


