Abstract
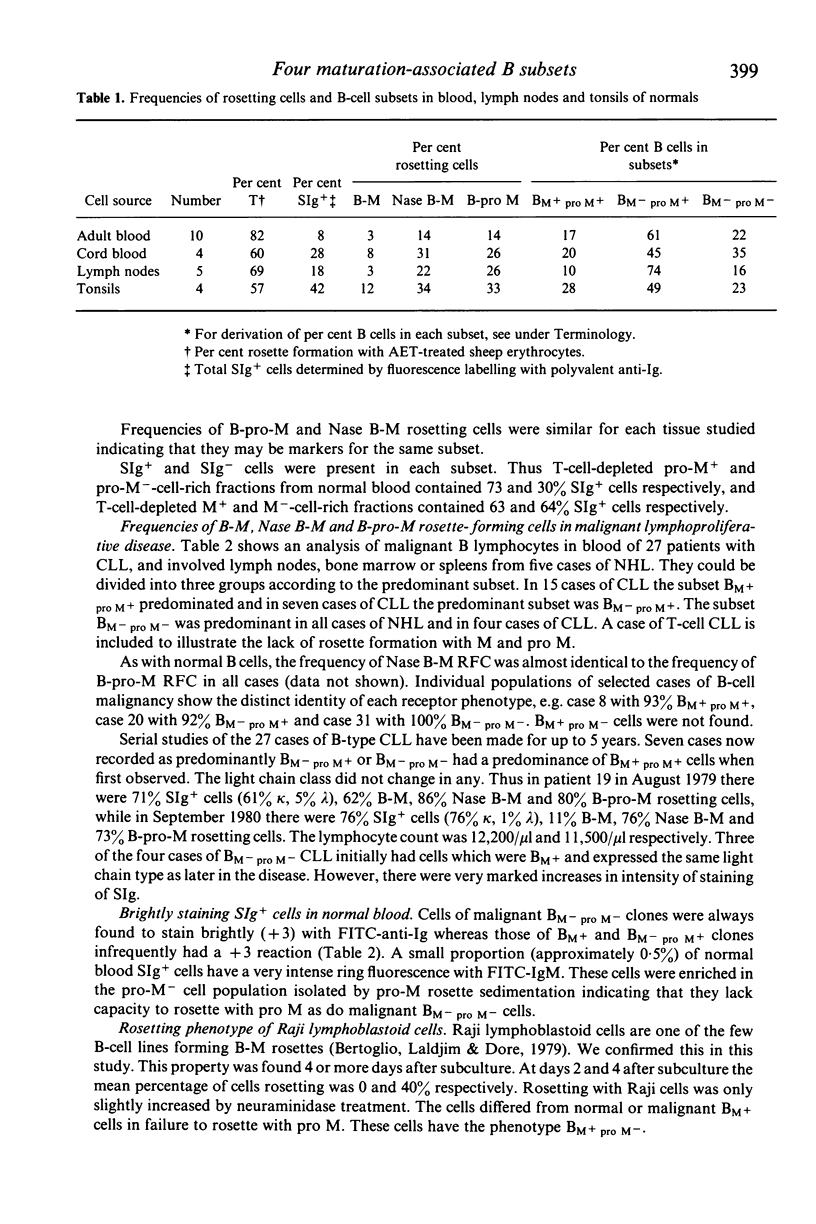
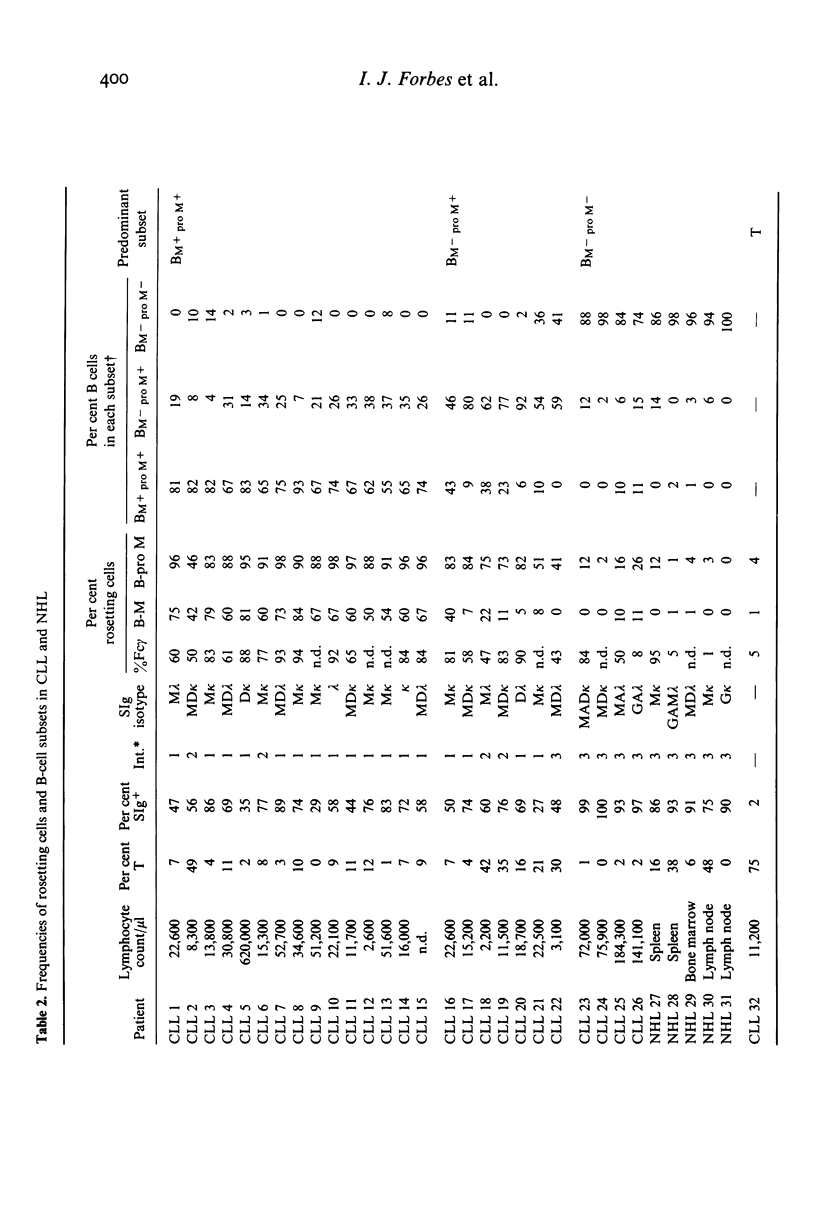
Using rosetting tests with untreated mouse erythrocytes (M) and pronase-treated M (pro M), four human B cell subsets can be identified. Three of these, possessing the phenotypes BM+ pro M+, BM- pro M+ or BM- pro M-, constitute 17%, 61% and 22% of normal blood B cells respectively. The fourth subset, BM+ pro M-, does not occur in normal tissues but was found in the pre-B-cell line of Raji cells, indicating that this phenotype may be a marker for early B cells. Some differences in the proportion of each subset were found in cord blood, lymph nodes and tonsils. Surface-immunoglobulin-positive (SIg+) and -negative (SIg-) non-T cells were present in each subset. M and pro-M rosetting tests were applied to cells from blood of 27 cases of chronic lymphocytic leukaemia (CLL) and to cells from involved nodes, spleen or marrow in five cases of non-Hodgkin's lymphoma (NHL). In 15 cases of CLL, there was considerable increase in the BM+ pro M+ subset (BM+ pro M+ type CLL); in seven cases, there was a predominance of BM- pro M+ cells and in another four cases, BM- pro M- cells predominated. All five cases of NHL were greatly enriched in BM- pro M- cells. There was no obvious correlation between rosetting and other surface markers but BM- pro M- clones in CLL or NHL always stained brightly with FITC-anti-Ig. This was not found in BM+ pro M+ or BM- pro M+ clones. Rosette formation of neuraminidase-treated B cells with M identifies the same subset as B-pro-M rosetting in normals and CLL. Evidence is presented that two types of receptors are involved in M and pro-M rosetting, designated R1 and R2, binding to corresponding M ligands L1 and L2. M rosetting is due to R1-L1 binding while R2-L2 binding mediates B-pro-M rosetting. Shifts between subsets within the same clone in some cases of CLL suggest that the subsets are distinct maturational stage of B-cell development rather than families of B cells of different lineage. The following B-cell maturation sequence is proposed: R1+ R2- lead to R1+ R2+ leads to R1- R2+ leads to R1- R2-.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertoglio J., Laldjim B., Doré J. F. Mouse erythrocyte rosette formation by human lymphoid cell lines. Scand J Immunol. 1979;10(2):153–159. doi: 10.1111/j.1365-3083.1979.tb03270.x. [DOI] [PubMed] [Google Scholar]
- Black R. B., Leong A. S., Cowled P. A., Forbes I. J. Lymphocyte subpopulations in human lymph nodes: a normal range. Lymphology. 1980 Jun;13(2):86–90. [PubMed] [Google Scholar]
- Catovsky D., Cherchi M., Okos A., Hegde U., Galton D. A. Mouse red-cell rosettes in B-lymphoproliferative disorders. Br J Haematol. 1976 Jun;33(2):173–177. doi: 10.1111/j.1365-2141.1976.tb03528.x. [DOI] [PubMed] [Google Scholar]
- Cordier G., Samarut C., Revillard J. P. Changes of Fcgamma receptor-related properties induced by interaction of human lymphocytes with insoluble immune complexes. J Immunol. 1977 Dec;119(6):1943–1948. [PubMed] [Google Scholar]
- Forbes I. J., Zalewski P. D. A subpopulation of human B lymphocytes that rosette with mouse erythrocytes. Clin Exp Immunol. 1976 Oct;26(1):99–107. [PMC free article] [PubMed] [Google Scholar]
- Forbes I. J., Zalewski P. D., Cowled P. A., Sage R. E. Maturation in B lymphocytic leukaemia. J Clin Lab Immunol. 1979 Feb;1(4):329–331. [PubMed] [Google Scholar]
- Forbes I. J., Zalewski P. D., Leong A. S., Sage R. E., Dale B., Cowled P. A. B cell leukaemia distinguished from chronic lymphocytic leukaemia by surface markers. Aust N Z J Med. 1978 Oct;8(5):532–538. doi: 10.1111/j.1445-5994.1978.tb02595.x. [DOI] [PubMed] [Google Scholar]
- Gupta S., Good R. A., Siegal F. P. Rosette-formation with mouse erythrocytes. II. A marker for human B and non-T lymphocytes. Clin Exp Immunol. 1976 Aug;25(2):319–327. [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Pahwa R., O'Reilly R., Good R. A., Siegal F. P. Ontogeny of lymphocyte subpopulations in human fetal liver. Proc Natl Acad Sci U S A. 1976 Mar;73(3):919–922. doi: 10.1073/pnas.73.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong A. S., Forbes I. J., Cowled P. A., Sage R. E., Black R. B., Dale B., Zalewski P. D. Surface marker studies in chronic lymphocytic leukaemia and non-Hodgkin's lymphoma. Pathology. 1979 Jul;11(3):461–471. doi: 10.3109/00313027909059023. [DOI] [PubMed] [Google Scholar]
- Salmon S. E., Seligmann M. B-cell neoplasia in man. Lancet. 1974 Nov 23;2(7891):1230–1233. doi: 10.1016/s0140-6736(74)90748-x. [DOI] [PubMed] [Google Scholar]
- Zalewski P. D., Forbes I. J. Studies of the human lymphocyte-mouse erythrocyte bond. Clin Exp Immunol. 1979 Jun;36(3):536–546. [PMC free article] [PubMed] [Google Scholar]
- Zola H. Fractionation of human lymphocytes using rosette formation with papain-treated mouse erythrocytes. J Immunol Methods. 1977;18(3-4):387–389. doi: 10.1016/0022-1759(77)90193-4. [DOI] [PubMed] [Google Scholar]


