Abstract
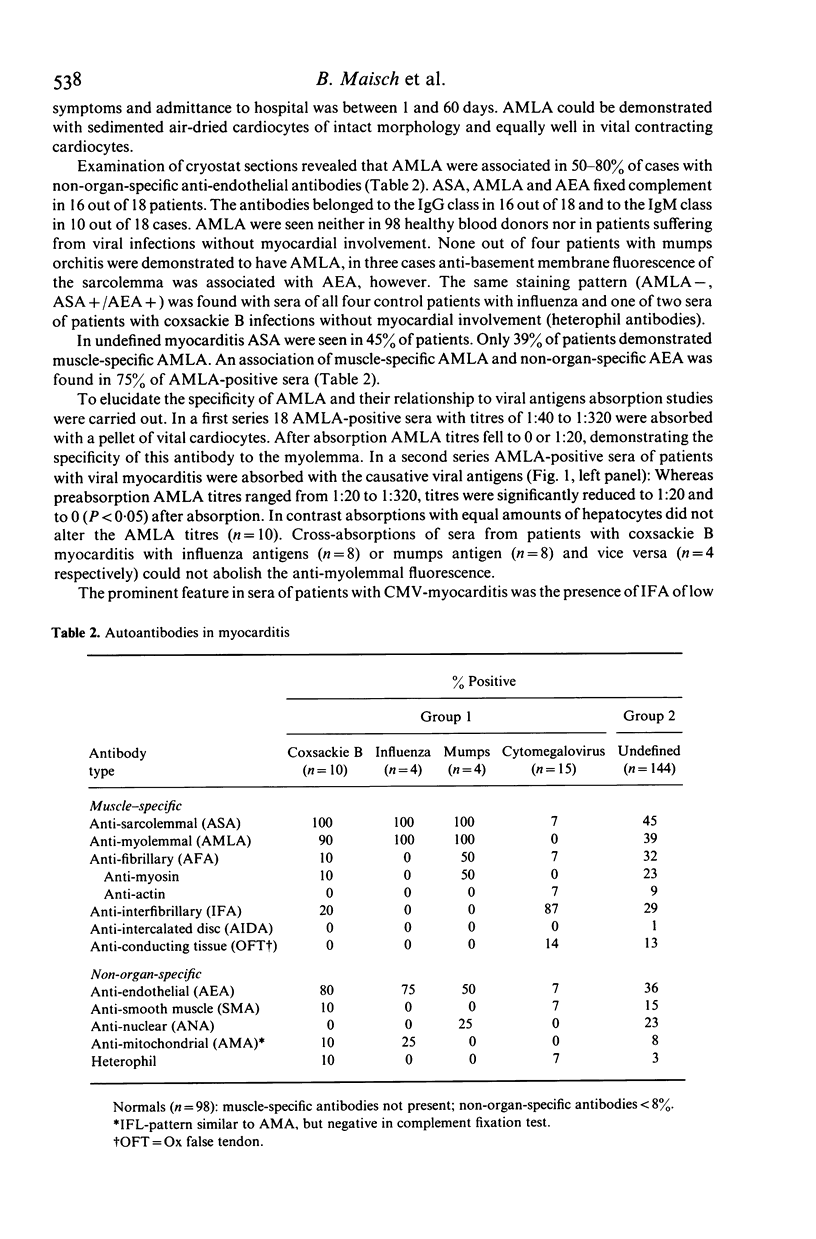
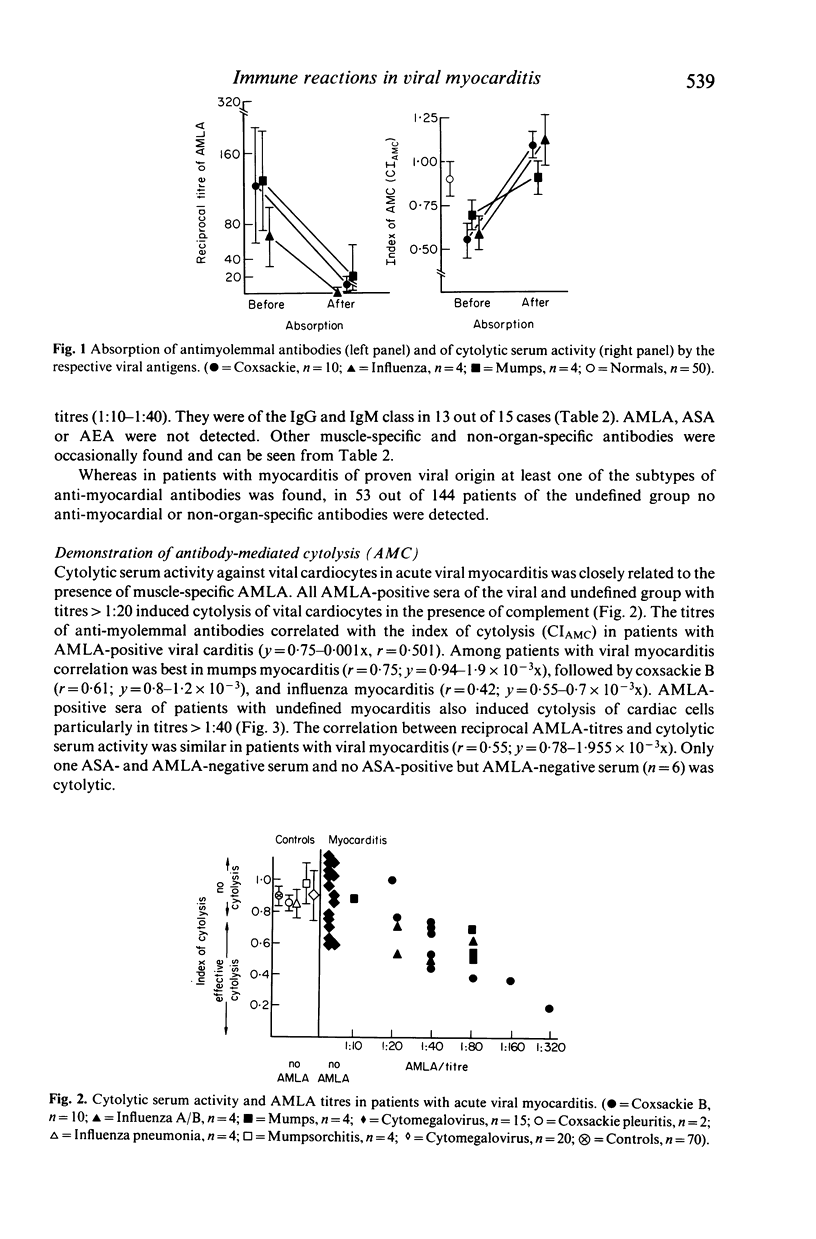
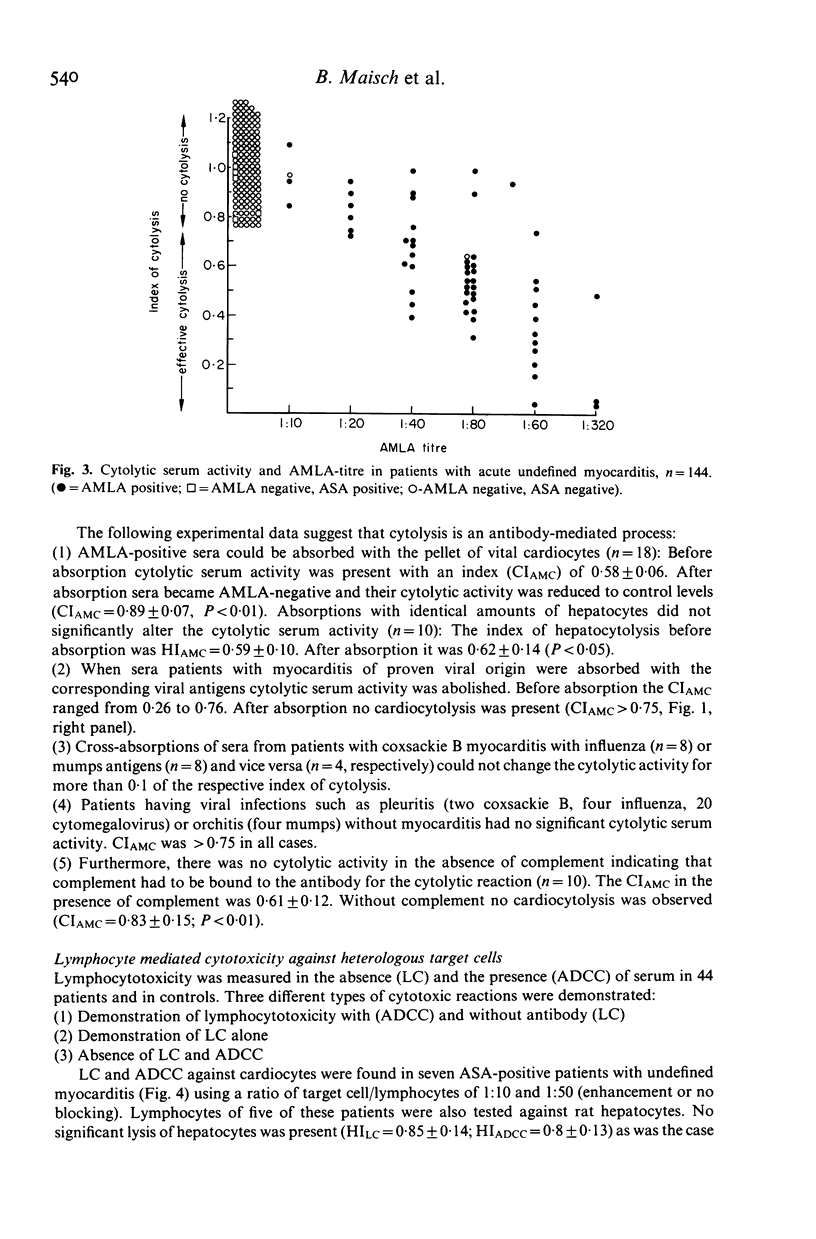
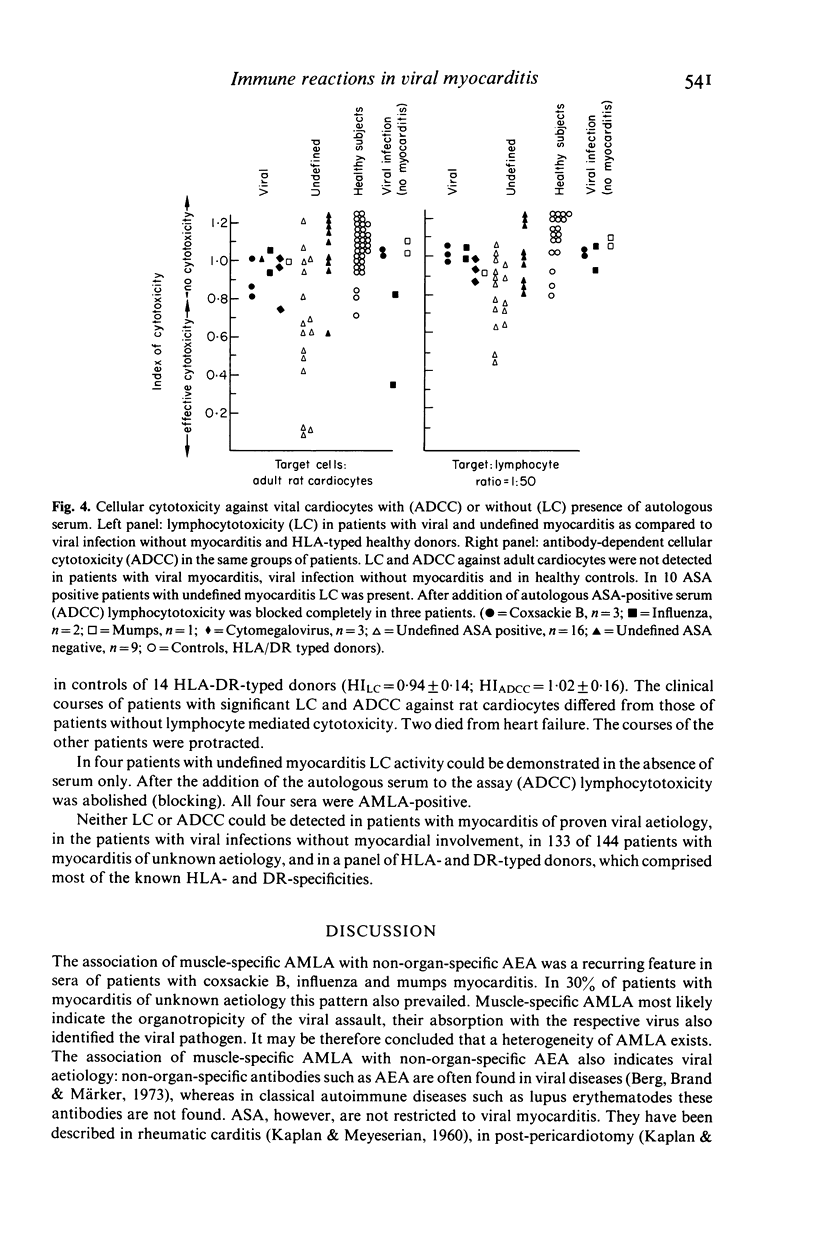
Sera of 177 patients with acute myocarditis (10 coxsackie B 3/4, four influenza, four mumps, 15 cytomegalovirus, 144 undefined) were tested by indirect immunofluorescence for autoantibodies against heart and skeletal muscle and vital or air-dried adult cardiocytes. Antibody-dependent cytolysis, lymphocytotoxicity and antibody-dependent cellular lymphocytotoxicity were assessed using viral adult rat cardiocytes as target cells. Muscle-specific anti-sarcolemmal antibodies of the anti-myolemmal type--often associated with non-organ-specific anti-endothelial antibodies--were demonstrated in nine out of 10 patients with coxsackie B, in all patients with influenza and mumps and in 65 out of 144 patients with undefined myocarditis. In contrast, 13 out of 15 patients with cytomegalovirus myocarditis lacked anti-sarcolemmal antibodies but had low titre anti-inter fibrillary antibodies instead. In the presence of complement, anti-myolemmal antibodies induced cytolysis of vital cardiocytes, whereas hepatocytes remained unaffected. Titres of anti-myolemmal antibodies correlated with the degree of cardiocytolysis. The anti-myolemmal immunofluorescent pattern and the cytolytic serum activity could be absorbed with the respective viral antigens suggesting that these antibodies cross-react with moieties of the virus itself and may be both diagnostic and aetiological markers in acute viral myocarditis. Lymphocyte-mediated cytotoxicity against heterologous cardiac target cells could not be observed in our patients with myocarditis of proven viral aetiology. However, lymphocyte-mediated cytotoxicity was demonstrated in 10 ASA-positive and one ASA-negative patient with myocarditis of unknown origin. ASA-positive sera blocked lymphocytotoxicity in three of these patients.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENGMARK S., FRISEN L., HELANDER E. A CYTOTOXIC SERUM FACTOR IN HEART DISEASE, ESPECIALLY MYOCARDITIS. Acta Allergol. 1963;18:479–488. doi: 10.1111/j.1398-9995.1963.tb03215.x. [DOI] [PubMed] [Google Scholar]
- Biddison W. E., Doherty P. C., Webster R. G. Antibody to influenza virus matrix protein detects a common antigen on the surface of cells infected with type A influenza viruses. J Exp Med. 1977 Sep 1;146(3):690–697. doi: 10.1084/jem.146.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch G. E., Giles T. D. The role of viruses in the production of heart disease. Am J Cardiol. 1972 Feb;29(2):231–240. doi: 10.1016/0002-9149(72)90634-0. [DOI] [PubMed] [Google Scholar]
- COONS A. H., KAPLAN M. H. Localization of antigen in tissue cells; improvements in a method for the detection of antigen by means of fluorescent antibody. J Exp Med. 1950 Jan 1;91(1):1–13. doi: 10.1084/jem.91.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragatakis L. N., Klassen J., Hüttner I., Fraser D. G., Poirier N. L., Klassen G. A. Autoimmune myocarditis: a clinical entity. Can Med Assoc J. 1979 Feb 3;120(3):317–321. [PMC free article] [PubMed] [Google Scholar]
- Ewan P. W., Lachmann P. J. Demonstration of T-cell and K-cell cytotoxicity against measles-infected cells in normal subjects, multiple sclerosis and subacute sclerosing panencephalitis. Clin Exp Immunol. 1977 Oct;30(1):22–31. [PMC free article] [PubMed] [Google Scholar]
- Ghose T., Mammen M. Interaction in vitro between myocardial cells and autologous lymphocytes and sera from patients with rheumatic carditis. Chest. 1977 Jun;71(6):730–734. doi: 10.1378/chest.71.6.730. [DOI] [PubMed] [Google Scholar]
- Greenberg S. B., Criswell B. S., Couch R. B. Lymohocyte-mediated cytotoxicity against influenza virus-infected cells: an in vitro method. J Immunol. 1975 Aug;115(2):601–603. [PubMed] [Google Scholar]
- Hawkins B. R., McDonald B. L., Dawkins R. L. Characterisation of immunofluorescent heterophile antibodies which may be confused with autoantibodies. J Clin Pathol. 1977 Apr;30(4):299–307. doi: 10.1136/jcp.30.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härfast B., Andersson T., Perlmann P. Immunoglobulin-independent natural cytotoxicity of Fc receptor-bearing human blood lymphocytes to mumps virus-infected target cells. J Immunol. 1978 Aug;121(2):755–761. [PubMed] [Google Scholar]
- Kaplan M. H., Frengley J. D. Autoimmunity to the heart in cardiac disease. Current concepts of the relation of autoimmunity to rheumatic fever, postcardiotomyand postinfarction syndromes and cardiomyopathies. Am J Cardiol. 1969 Oct;24(4):459–473. doi: 10.1016/0002-9149(69)90489-5. [DOI] [PubMed] [Google Scholar]
- Laufer A. pathogenesis."Pathogenesis. Isr J Med Sci. 1975 Jan;11(1):37–66. [PubMed] [Google Scholar]
- Liu M. S., Spitzer J. J. Oxidation of palmitate and lactate by beating myocytes isolated from adult dog heart. J Mol Cell Cardiol. 1978 May;10(5):415–426. doi: 10.1016/0022-2828(78)90363-2. [DOI] [PubMed] [Google Scholar]
- Longson M., Cole F. M., Davies D. Isolation of a Coxsackie virus group B, type 5, from the heart of a fatal case of myocarditis in an adult. J Clin Pathol. 1969 Nov;22(6):654–658. doi: 10.1136/jcp.22.6.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisch B., Berg P. A., Kochsiek K. Autoantibodies and serum inhibition factors (sif) in patients with myocarditis. Klin Wochenschr. 1980 Mar 3;58(5):219–225. doi: 10.1007/BF01476967. [DOI] [PubMed] [Google Scholar]
- Maisch B., Berg P. A., Kochsiek K. Clinical significance of immunopathological findings in patients with post-pericardiotomy syndrome. I. Relevance of antibody pattern. Clin Exp Immunol. 1979 Nov;38(2):189–197. [PMC free article] [PubMed] [Google Scholar]
- Maisch B., Berg P. A., Kochsiek K. Immunological parameters in patients with congestive cardiomyopathy. Basic Res Cardiol. 1980 Jan-Feb;75(1):221–222. doi: 10.1007/BF02001417. [DOI] [PubMed] [Google Scholar]
- Maisch B. Enrichment of vital adult cardiac muscle cells by continuous silica sol gradient centrifugation. Basic Res Cardiol. 1981 Nov-Dec;76(6):622–629. doi: 10.1007/BF01908052. [DOI] [PubMed] [Google Scholar]
- Maisch B., Schuff-Werner P., Berg P. A., Kochsiek K. Clinical significance of immunopathological findings in patients with post-pericardiotomy syndrome. II. The significance of serum inhibition and rosette inhibitory factors. Clin Exp Immunol. 1979 Nov;38(2):198–203. [PMC free article] [PubMed] [Google Scholar]
- Maisch B., Trostel-Soeder R., Berg P. A., Kochsiek K. Assessment of antibody mediated cytolysis of adult cardiocytes isolated by centrifugation in a continuous gradient of Percoll in patients with acute myocarditis. J Immunol Methods. 1981;44(2):159–169. doi: 10.1016/0022-1759(81)90343-4. [DOI] [PubMed] [Google Scholar]
- Masson-Pévet M., Jongsma H. J., De Bruijne J. Collagenase- and trypsin-dissociated heart cells: a comparative ultrastructural study. J Mol Cell Cardiol. 1976 Oct;8(10):747–757. doi: 10.1016/0022-2828(76)90082-1. [DOI] [PubMed] [Google Scholar]
- Masucci M. G., Klein E., Argov S. Disappearance of the NK effect after explantation of lymphocytes and generation of similar nonspecific cytotoxicity correlated to the level of blastogenesis in activated cultures. J Immunol. 1980 May;124(5):2458–2463. [PubMed] [Google Scholar]
- Paque R. E., Gauntt C. J., Nealon T. J., Trousdale M. D. Assessment of cell-mediated hypersensitivity against coxsackievirus B3 viral-induced myocarditis utilizing hypertonic salt extracts of cardiac tissue. J Immunol. 1978 May;120(5):1672–1678. [PubMed] [Google Scholar]
- Powell T., Twist V. W. A rapid technique for the isolation and purification of adult cardiac muscle cells having respiratory control and a tolerance to calcium. Biochem Biophys Res Commun. 1976 Sep 7;72(1):327–333. doi: 10.1016/0006-291x(76)90997-9. [DOI] [PubMed] [Google Scholar]
- Rager-Zisman B., Allison A. C. The role of antibody and host cells in the resistance of mice against infection by coxsackie B-3 virus. J Gen Virol. 1973 Jun;19(3):329–338. doi: 10.1099/0022-1317-19-3-329. [DOI] [PubMed] [Google Scholar]
- Singh J. N., Sabbadini E., Sehon A. H. Detection of nonspecific cytotoxicity in graft-versus-host reaction as a function of target cell type. Cell Immunol. 1973 Aug;8(2):280–289. doi: 10.1016/0008-8749(73)90117-2. [DOI] [PubMed] [Google Scholar]
- Sládková T., Stefan J., Bícová R. Srdecní protilátky v diagnostice zánetu srdecního svalu. Cas Lek Cesk. 1979 Nov 9;118(45):1395–1398. [PubMed] [Google Scholar]
- Steele R. W., Vincent M. M., Hensen S. A., Fuccillo D. A., Chapa I. A., Canales L. Cellular immune responses to Herpes simplex virus type 1 in recurrent herpes labialis: in vitro blastogenesis and cytotoxicity to infected cell line. J Infect Dis. 1975 May;131(5):528–534. doi: 10.1093/infdis/131.5.528. [DOI] [PubMed] [Google Scholar]
- Thompson A., Halbert S. P. The cardiac auto-immune system. 3. Studies on the cytotoxicity of heart antibodies for pulsating rabbit and rat heart cells in tissue culture. Int Arch Allergy Appl Immunol. 1971;40(2):274–286. [PubMed] [Google Scholar]
- Thong Y. H., Hensen S. A., Vincent M. M., Fuccillo D. A., Stiles W. A., Bellanti J. A. Use of cryopreserved virus-infected target cells in a lymphocytotoxicity 51Cr release microassay for cell-mediated immunity to cytomegalovirus. Infect Immun. 1976 Feb;13(2):643–645. doi: 10.1128/iai.13.2.643-645.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M., Jr, Hallenbeck L. A. Effect of virus infections on target cell susceptibility to natural killer cell-mediated lysis. J Immunol. 1980 May;124(5):2491–2497. [PubMed] [Google Scholar]
- Wong C. Y., Woodruff J. J., Woodruff J. F. Generation of cytotoxic T lymphocytes during coxsackievirus B-3 infection. I. Model and viral specificity1. J Immunol. 1977 Apr;118(4):1159–1164. [PubMed] [Google Scholar]
- Woodruff J. F., Woodruff J. J. Involvement of T lymphocytes in the pathogenesis of coxsackie virus B3 heart disease. J Immunol. 1974 Dec;113(6):1726–1734. [PubMed] [Google Scholar]
- Yang L. C., Soprey P. R., Wittner M. K., Fox E. N. Streptococcal-induced cell-mediated-immune destruction of cardiac myofibers in vitro. J Exp Med. 1977 Aug 1;146(2):344–360. doi: 10.1084/jem.146.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]


