Abstract
Hypergammaglobulinaemia (HGG) is frequently found in patients with hepatic cirrhosis (HC). Using an assay system of in vitro PWM-stimulated immunoglobulin (Ig) production, the amounts of IgG, IgA, and IgM produced by peripheral blood lymphocytes (PBL) from 15 HBs Ag-negative patients with HC and from 16 age-matched healthy subjects were quantitated by radioimmunoassay. We found that PBL from patients with HC produced significantly greater amounts of IgG (P less than 0.05) but not IgA or IgM than did those from control subjects. This increased IgG production by PBL from patients with HC was attributed to enhanced T helper activity and not to enhanced B cell function. We also searched for defects in naturally occurring suppressor T cell activity which is sensitive to irradiation. Irradiation-induced enhancement for IgG production was significantly lower in patients with HC compared with age-matched control subjects (P less than 0.01). Similarly, we examined the effect of Con A-induced suppressor T cells on the in vitro PWM-stimulated IgG production by allogeneic PBL and observed the decrease of Con A-induced suppressor T cell activity in patients with HC (P = 0.01). We conclude, therefore, that the increased serum levels of Ig, particularly IgG in patients with HC may result from in part on the basis of depressed ability of naturally occurring suppressor T cells or Con A-induced suppressor T cells to suppress Ig production.
Full text
PDF



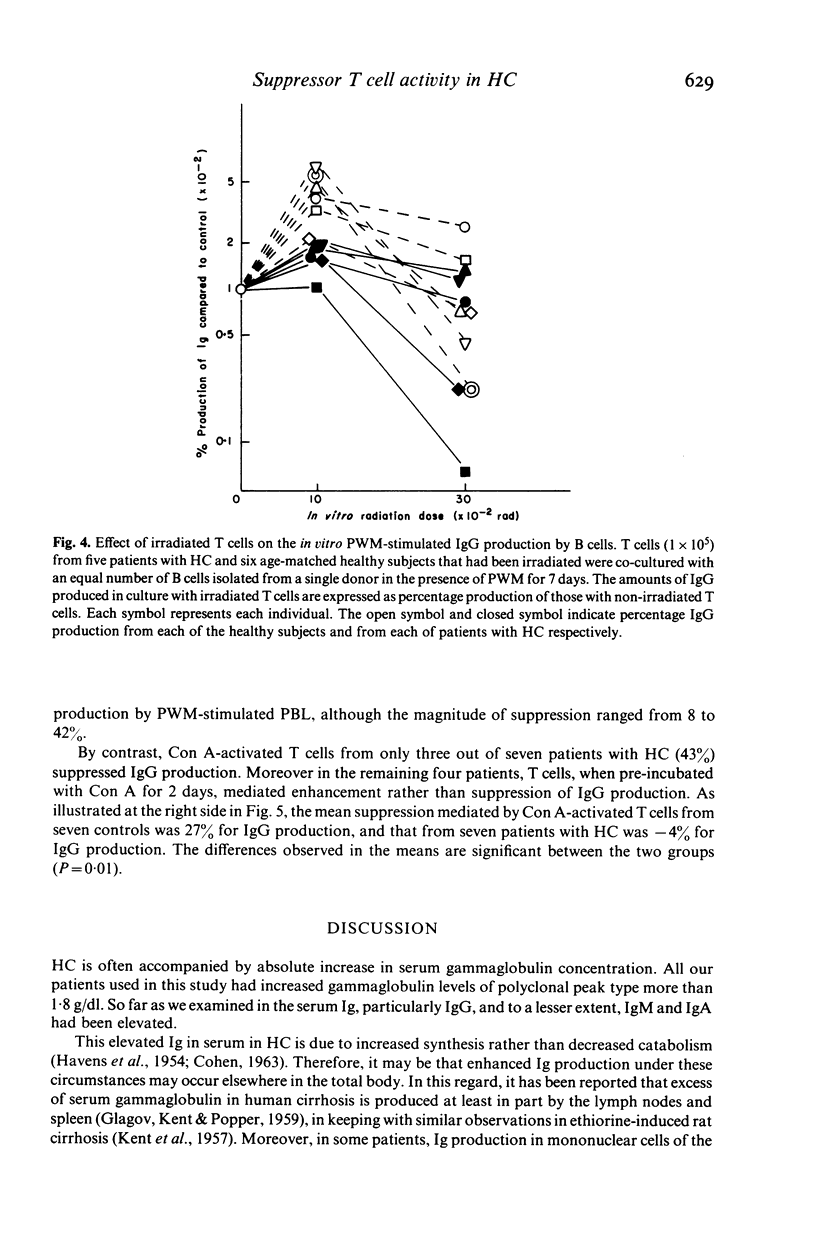
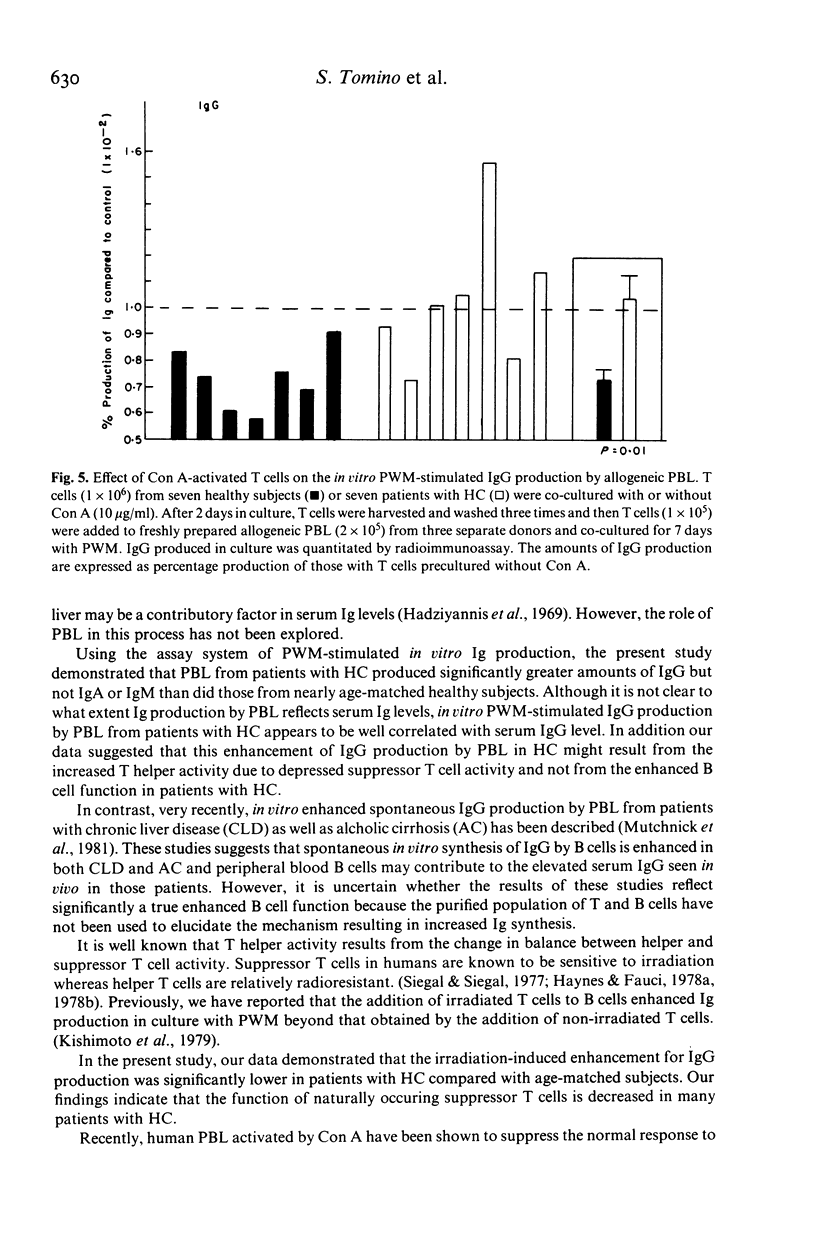


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjorneboe M., Prytz H., Orskov F. Antibodies to intestinal microbes in serum of patients with cirrhosis of the liver. Lancet. 1972 Jan 8;1(7741):58–60. doi: 10.1016/s0140-6736(72)90060-8. [DOI] [PubMed] [Google Scholar]
- COHEN S. GAMMA-GLOBULIN METABOLISM. Br Med Bull. 1963 Sep;19:202–206. doi: 10.1093/oxfordjournals.bmb.a070057. [DOI] [PubMed] [Google Scholar]
- Colombo M., Vernace S. J., Paronetto F. T and B lymphocytes in patients with chronic active hepatitis (CAH). Clin Exp Immunol. 1977 Oct;30(1):4–9. [PMC free article] [PubMed] [Google Scholar]
- Doniach D., Roitt I. M., Walker J. G., Sherlock S. Tissue antibodies in primary biliary cirrhosis, active chronic (lupoid) hepatitis, cryptogenic cirrhosis and other liver diseases and their clinical implications. Clin Exp Immunol. 1966 Jul;1(3):237–262. [PMC free article] [PubMed] [Google Scholar]
- GLAGOV S., KENT G., POPPER H. Relation of splenic and lymph node changes to hypergammaglobulinemia in cirrhosis. AMA Arch Pathol. 1959 Jan;67(1):9–18. [PubMed] [Google Scholar]
- HAVENS W. P., Jr, DICKENSHEETS J., BIERLY J. N., EBERHARD T. P. The half-life of I131 labeled normal human gamma globulin in patients with hepatic cirrhosis. J Immunol. 1954 Oct;73(4):256–258. [PubMed] [Google Scholar]
- Hadziyannis S., Feizi T., Scheuer P. J., Sherlock S. Immunoglobulin-containing cells in the liver. Clin Exp Immunol. 1969 Nov;5(5):499–514. [PMC free article] [PubMed] [Google Scholar]
- Hallgren H. M., Yunis E. J. Suppressor lymphocytes in young and aged humans. J Immunol. 1977 Jun;118(6):2004–2008. [PubMed] [Google Scholar]
- Haynes B. F., Fauci A. S. Activation of human B lymphocytes. III. Concanavalin A-induced generation of suppressor cells of the plaque-forming cell response of normal human B lymphocytes. J Immunol. 1977 Jun;118(6):2281–2287. [PubMed] [Google Scholar]
- Haynes B. F., Fauci A. S. Activation of human B lymphocytes. V. Kinetics and mechanisms of suppression of plaque-forming cell responses by concanavalin A-generated suppressor cells. J Immunol. 1978 Mar;120(3):700–708. [PubMed] [Google Scholar]
- Haynes B. F., Fauci A. S. Activation of human B lymphocytes. VI. Immunoregulation of antibody production by mitogen-induced and naturally occurring cells in normal individuals. Cell Immunol. 1978 Mar 15;36(2):294–302. doi: 10.1016/0008-8749(78)90273-3. [DOI] [PubMed] [Google Scholar]
- KENT G., POPPER H., DUBIN A., BRUCE C. The spleen in ethionine-induced cirrhosis; its role in gama-globulin elevation. AMA Arch Pathol. 1957 Oct;64(4):398–408. [PubMed] [Google Scholar]
- Kishimoto S., Tomino S., Inomata K., Kotegawa S., Saito T., Kuroki M., Mitsuya H., Hisamitsu S. Age-related changes in the subsets and functions of human T lymphocytes. J Immunol. 1978 Nov;121(5):1773–1780. [PubMed] [Google Scholar]
- Kishimoto S., Tomino S., Mitsuya H., Fujiwara H. Age-related changes in suppressor functions of human T cells. J Immunol. 1979 Oct;123(4):1586–1593. [PubMed] [Google Scholar]
- McFarlane I. G., Eddleston A. L., Williams R. Lymphocyte subpopulations in chronic liver disease. Clin Exp Immunol. 1977 Oct;30(1):1–3. [PMC free article] [PubMed] [Google Scholar]
- Mutchnick M. G., Lederman H. M., Missirian A., Johnson A. G. In vitro synthesis of IgG by peripheral blood lymphocytes in chronic liver disease. Clin Exp Immunol. 1981 Feb;43(2):370–375. [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. Con A-inducible suppression of MLC: evidence for mediation by the TH2 + T cell subset in man. J Immunol. 1979 Apr;122(4):1335–1341. [PubMed] [Google Scholar]
- Sakane T., Green I. Human suppressor T cells induced by concanavalin A: suppressor T cells belong to distinctive T cell subclasses. J Immunol. 1977 Sep;119(3):1169–1178. [PubMed] [Google Scholar]
- Shou L., Schwartz S. A., Good R. A. Suppressor cell activity after concanavalin A treatment of lymphocytes from normal donors. J Exp Med. 1976 May 1;143(5):1100–1110. doi: 10.1084/jem.143.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal F. P., Siegal M. Enhancement by irradiated T cells of human plasma cell production: dissection of helper and suppressor functions in vitro. J Immunol. 1977 Feb;118(2):642–647. [PubMed] [Google Scholar]
- Triger D. R., Alp M. H., Wright R. Bacterial and dietary antibodies in liver disease. Lancet. 1972 Jan 8;1(7741):60–63. doi: 10.1016/s0140-6736(72)90061-x. [DOI] [PubMed] [Google Scholar]
- Triger D. R., Wright R. Hyperglobulinaemia in liver disease. Lancet. 1973 Jun 30;1(7818):1494–1496. doi: 10.1016/s0140-6736(73)91827-8. [DOI] [PubMed] [Google Scholar]


