Abstract
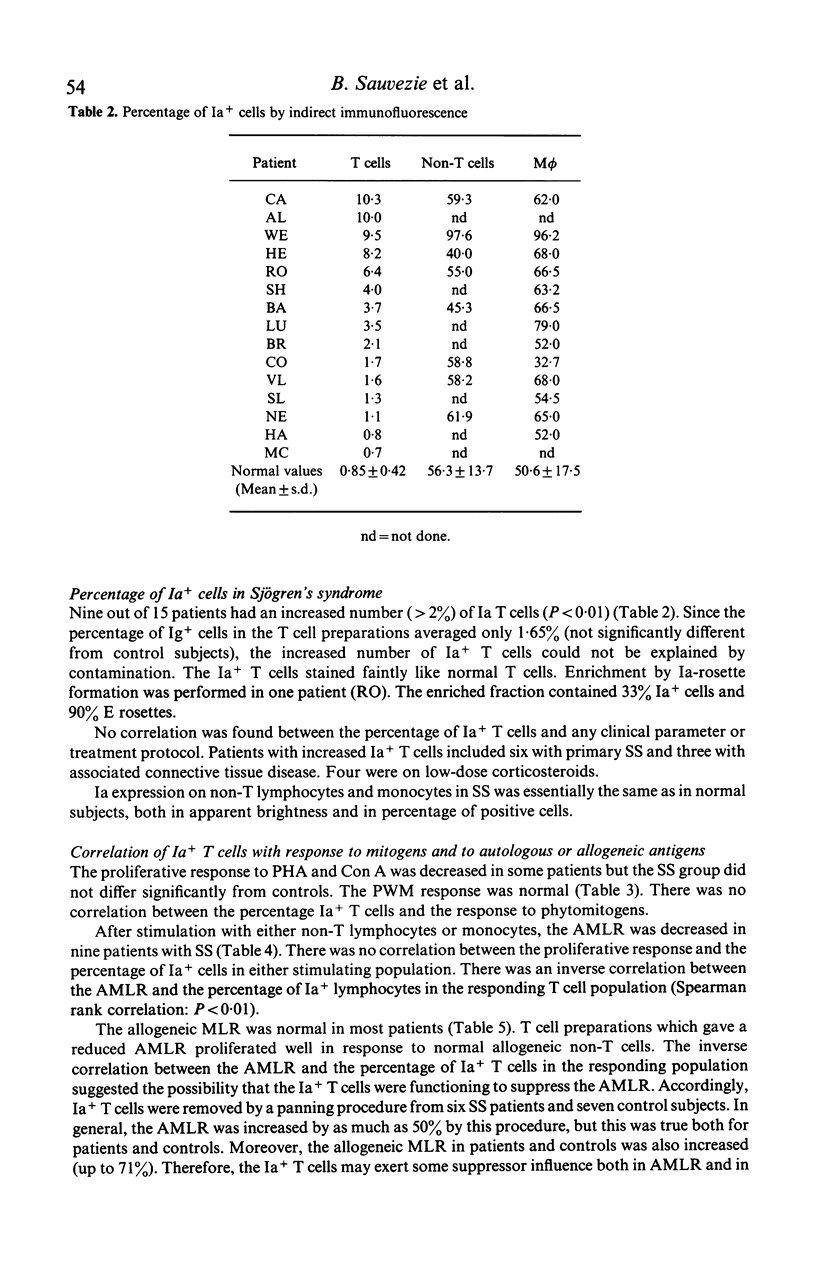
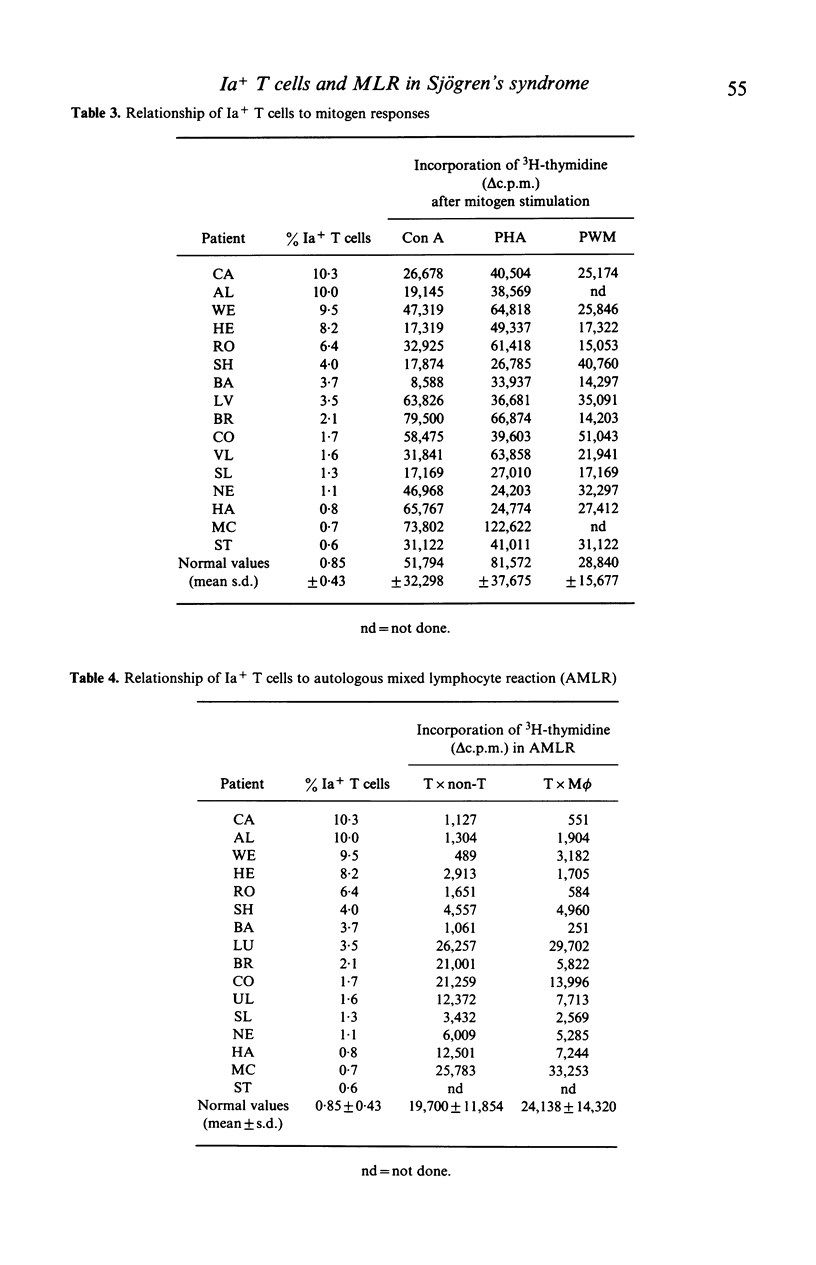
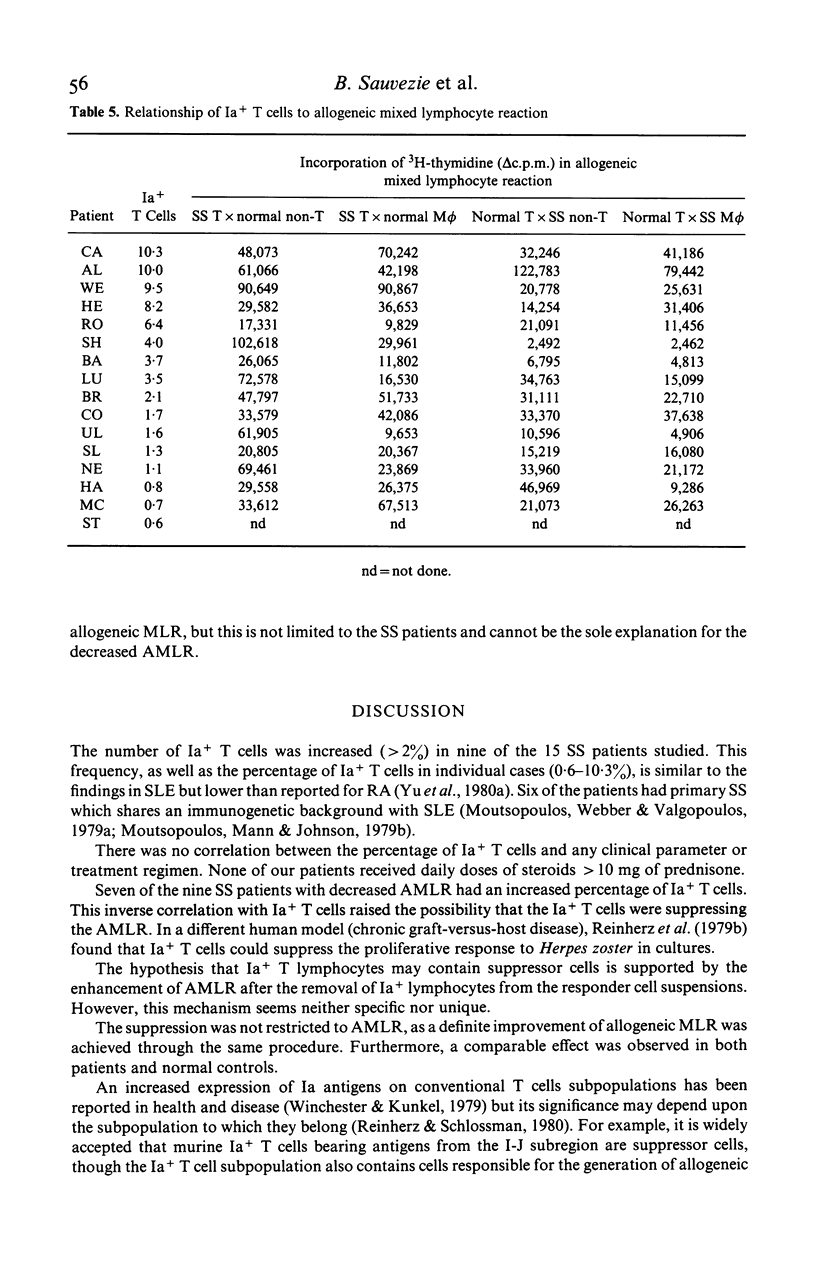
The defective autologous MLR was studied in Sjögren's syndrome (SS) in relation to Ia+ cells as determined by reactivity with a monoclonal anti-human Ia antibody. By indirect immunofluorescence, the percentage of Ia+ T lymphocytes was increased in nine of 15 patients. There was no correlation with clinical features or drugs. The percentage of Ia+ T cells in the non-T cell preparations was normal. An inverse correlation was found between the percentage of Ia+ T cells and the proliferative response to autologous non-T cells. Removal of Ia+ T cells enhanced both the autologous MLR and the allogeneic MLR. Thus Ia+ T cells contain suppressor cells in the MLR, but this may not be the sole explanation for the defective autologous MLR.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLOCH K. J., BUCHANAN W. W., WOHL M. J., BUNIM J. J. SJOEGREN'S SYNDROME. A CLINICAL, PATHOLOGICAL, AND SEROLOGICAL STUDY OF SIXTY-TWO CASES. Medicine (Baltimore) 1965 May;44:187–231. [PubMed] [Google Scholar]
- Charron D. J., McDevitt H. O. Analysis of HLA-D region-associated molecules with monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6567–6571. doi: 10.1073/pnas.76.12.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels T. E., Silverman S., Jr, Michalski J. P., Greenspan J. S., Sylvester R. A., Talal N. The oral component of Sjögren's syndrome. Oral Surg Oral Med Oral Pathol. 1975 Jun;39(6):875–885. doi: 10.1016/0030-4220(75)90108-5. [DOI] [PubMed] [Google Scholar]
- Engleman E. G., Benike C. J., Charron D. J. Ia antigen on peripheral blood mononuclear leukocytes in man. II. Functional studies of HLA-DR-positive T cells activated in mixed lymphocyte reactions. J Exp Med. 1980 Aug 1;152(2 Pt 2):114s–126s. [PubMed] [Google Scholar]
- Engleman E. G., Benike C. J., Hoppe R. T., Kaplan H. S., Berberich F. R. Autologous mixed lymphocyte reaction in patients with Hodgkin's disease. Evidence for a T cell defect. J Clin Invest. 1980 Jul;66(1):149–158. doi: 10.1172/JCI109828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman E. G., Charron D. J., Benike C. J., Stewart G. J. Ia antigen on peripheral blood mononuclear leukocytes in man. I. Expression, biosynthesis, and function of HLA-DR antigen non-T cells. J Exp Med. 1980 Aug 1;152(2 Pt 2):99s–113s. [PubMed] [Google Scholar]
- Evans R. L., Faldetta T. J., Humphreys R. E., Pratt D. M., Yunis E. J., Schlossman S. F. Peripheral human T cells sensitized in mixed leukocyte culture synthesize and express Ia-like antigens. J Exp Med. 1978 Nov 1;148(5):1440–1445. doi: 10.1084/jem.148.5.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S. M., Chiorazzi N., Wang C. Y., Montrazeri G., Kunkel H. G., Ko H. S., Gottlieb A. B. Ia-bearing T lymphocytes in man. Their identification and role in the generation of allogeneic helper activity. J Exp Med. 1978 Nov 1;148(5):1423–1428. doi: 10.1084/jem.148.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher L. H., Steinberg A. D., House S. B., Green I. The autologous mixed lymphocyte reaction in strains of mice with autoimmune disease. J Immunol. 1980 Oct;125(4):1832–1838. [PubMed] [Google Scholar]
- Gottlieb A. B., Fu S. M., Yu D. T., Wang C. Y., Halper J. P., Kunkel H. G. The nature of the stimulatory cell in human allogeneic and autologous MLC reactions; role of isolated IgM-bearing B cells. J Immunol. 1979 Oct;123(4):1497–1503. [PubMed] [Google Scholar]
- Hausman P. B., Raff H. V., Gilbert R. C., Picker L. J., Stobo J. D. T cells and macrophages involved in the autologous mixed lymphocyte reaction are required for the response to conventional antigen. J Immunol. 1980 Sep;125(3):1374–1379. [PubMed] [Google Scholar]
- Hausman P. B., Stites D. P., Stobo J. D. Antigen-reactive T cells can be activated buy autologous macrophages in the absence of added antigen. J Exp Med. 1981 Feb 1;153(2):476–481. doi: 10.1084/jem.153.2.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaszubowski P. A., Goodwin J. S., Williams R. C., Jr Ia antigen on the surface of a subfraction of T cells that bear Fc receptors for IgG. J Immunol. 1980 Mar;124(3):1075–1078. [PubMed] [Google Scholar]
- Katz D. H., Graves M., Dorf M. E., Dimuzio H., Benacerraf B. Cell interactions between histoincompatible T and B lymphocytes. VII. Cooperative responses between lymphocytes are controlled by genes in the I region of the H-2 complex. J Exp Med. 1975 Jan 1;141(1):263–268. doi: 10.1084/jem.141.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H. S., Fu S. M., Winchester R. J., Yu D. T., Kunkel H. G. Ia determinants on stimulated human T lymphocytes. Occurrence on mitogen- and antigen-activated T cells. J Exp Med. 1979 Aug 1;150(2):246–255. doi: 10.1084/jem.150.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz M. M., Innes J. B., Weksler M. E. The cellular basis of the impaired autologous mixed lymphocyte reaction in patients with systemic lupus erythematosus. J Clin Invest. 1979 Jan;63(1):151–153. doi: 10.1172/JCI109270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaka N., Sauvezie B., Pierce D. A., Daniels T. E., Talal N. Decreased autologous mixed lymphocyte reaction in Sjögren's syndrome. J Clin Invest. 1980 Nov;66(5):928–933. doi: 10.1172/JCI109960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody C. E., Casazza B. A., Christenson W. N., Weksler M. E. Lymphocyte transformation induced by autologous cells. VIII. Impaired autologous mixed lymphocyte reactivity in patients with acute infectious mononucleosis. J Exp Med. 1979 Dec 1;150(6):1448–1455. doi: 10.1084/jem.150.6.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutsopoulos H. M., Mann D. L., Johnson A. H., Chused T. M. Genetic differences between primary and secondary sicca syndrome. N Engl J Med. 1979 Oct 4;301(14):761–763. doi: 10.1056/NEJM197910043011405. [DOI] [PubMed] [Google Scholar]
- Moutsopoulos H. M., Webber B. L., Vlagopoulos T. P., Chused T. M., Decker J. L. Differences in the clinical manifestations of sicca syndrome in the presence and absence of rheumatoid arthritis. Am J Med. 1979 May;66(5):733–736. doi: 10.1016/0002-9343(79)91110-0. [DOI] [PubMed] [Google Scholar]
- Niederhuber J. E., Frelinger J. A. Expression of Ia antigens on T and B cells and their relationship to immune-response functions. Transplant Rev. 1976;30:101–121. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Pesando J. M., Ritz J., Goldstein G., Schlossman S. F. Ia determinants on human T-cell subsets defined by monoclonal antibody. Activation stimuli required for expression. J Exp Med. 1979 Dec 1;150(6):1472–1482. doi: 10.1084/jem.150.6.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Parkman R., Rappeport J., Rosen F. S., Schlossman S. F. Aberrations of suppressor T cells in human graft-versus-host disease. N Engl J Med. 1979 May 10;300(19):1061–1068. doi: 10.1056/NEJM197905103001901. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. Current concepts in immunology: Regulation of the immune response--inducer and suppressor T-lymphocyte subsets in human beings. N Engl J Med. 1980 Aug 14;303(7):370–373. doi: 10.1056/NEJM198008143030704. [DOI] [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Green I. Failure of autologous mixed lymphocyte reactions between T and non-T cells in patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3464–3468. doi: 10.1073/pnas.75.7.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurman R. K., Gelfand E. W., Matheson D., Zimmerman B., Dosch H. M. Identification of Ia on a subpopulation of human T lymphocytes that stimulate in a mixed lymphocyte reaction. J Immunol. 1980 Apr;124(4):1924–1928. [PubMed] [Google Scholar]
- Smith J. B., Knowlton R. P., Koons L. S. Immunologic studies in chronic lymphocytic leukemia: defective stimulation of T-cell proliferation in autologous mixed lymphocyte culture. J Natl Cancer Inst. 1977 Mar;58(3):579–585. doi: 10.1093/jnci/58.3.579. [DOI] [PubMed] [Google Scholar]
- Winchester R. J., Wernet P., Kunkel H. G., Dupont B., Jersild C., Fu S. M. Recognition by pregnancy serums of non-HL-A alloantigens selectively expressed on B lymphocytes. J Exp Med. 1975 Apr 1;141(4):924–929. [PMC free article] [PubMed] [Google Scholar]
- Yu D. T., McCune J. M., Fu S. M., Winchester R. J., Kunkel H. G. Two types of Ia-positive T cells. Synthesis and exchange of Ia antigens. J Exp Med. 1980 Aug 1;152(2 Pt 2):89s–98s. [PubMed] [Google Scholar]
- Yu D. T., Winchester R. J., Fu S. M., Gibofsky A., Ko H. S., Kunkel H. G. Peripheral blood Ia-positive T cells. Increases in certain diseases and after immunization. J Exp Med. 1980 Jan 1;151(1):91–100. doi: 10.1084/jem.151.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]


