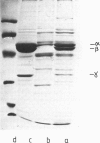
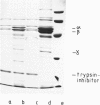
Abstract
The complement fixing antigen of the inner mitochondrial membrane previously shown to be associated with the mitochondrial ATPase could be further purified by subjecting the ATPase extracted from beef heart and brown fat mitochondria to ion exchange and gel filtration chromatography. Although the ATPase activity could be clearly dissociated from the complement fixing activity, subunits of the F1-ATPase complex were always found in the purified fractions. The alpha, gamma, delta and epsilon subunits of the complex could be excluded with high probability as target antigens in contrast to the beta band which was always found in association with the antigen activity. These findings imply that the active centre of the ATPase enzyme is not involved in the antibody reaction but molecules of the ATPase complex may have antigen binding capacity. Treatment of ATPase associated antigen with trypsin did not markedly affect the complement binding, while SMP's treated in the same way lost their antigen activity indicating that sera from patients with primary biliary cirrhosis (PBC) may have mitochondrial antibodies of different specificities reacting with trypsin sensitive as well as trypsin insensitive components of the inner membrane. The purified antigen reacted exclusively with sera from patients with PBC and may be therefore used as a marker antigen.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apps D. K., Schatz G. An adenosine triphosphatase isolated from chromaffin-granulate membranes is closely similar to F1-adenosine triphosphatase of mitochondria. Eur J Biochem. 1979 Oct 15;100(2):411–419. doi: 10.1111/j.1432-1033.1979.tb04184.x. [DOI] [PubMed] [Google Scholar]
- Baum H., Berg P. A. The complex nature of mitochondrial antibodies and their relation to primary biliary cirrhosis. Semin Liver Dis. 1981 Nov;1(4):309–321. doi: 10.1055/s-2008-1040734. [DOI] [PubMed] [Google Scholar]
- Beechey R. B., Hubbard S. A., Linnett P. E., Mitchell A. D., Munn E. A. A simple and rapid method for the preparation of adenosine triphosphatase from submitochondrial particles. Biochem J. 1975 Jun;148(3):533–537. doi: 10.1042/bj1480533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B., Vogel G. The mitochondrial ATPase of brown adipose tissue. Purification and comparison with the mitochondrial ATPase from beef heart. FEBS Lett. 1977 Apr 15;76(2):284–289. doi: 10.1016/0014-5793(77)80169-5. [DOI] [PubMed] [Google Scholar]
- Homberg J. C., Stelly N., Andreis I., Abuaf N., Saadoun F., Andre J. A new antimitochondria antibody (anti-M6) in iproniazid-induced hepatitis. Clin Exp Immunol. 1982 Jan;47(1):93–102. [PMC free article] [PubMed] [Google Scholar]
- Kopecký J., Houstek J., Drahota Z. Purification and properties of adenosine triphosphatase solubilized from beef heart mitochondria by chloroform. Mol Cell Biochem. 1977 Dec 29;18(2-3):77–80. doi: 10.1007/BF00280271. [DOI] [PubMed] [Google Scholar]
- Labro M. T., Andrieu M. C., Weber M., Homberg J. C. A new pattern of non-organ- and non-species-specific anti-organelle antibody detected by immunofluorescence: the mitochondrial antibody number 5. Clin Exp Immunol. 1978 Mar;31(3):357–366. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Meek F., Khoury E. L., Doniach D., Baum H. Mitochondrial antibodies in chronic liver diseases and connective tissue disorders: further characterization of the autoantigens. Clin Exp Immunol. 1980 Jul;41(1):43–54. [PMC free article] [PubMed] [Google Scholar]
- Miyachi K., Gupta R. C., Dickson E. R., Tan E. M. Preciitating antibodies to mitochondrial antigens in patients with primary biliary cirrhosis. Clin Exp Immunol. 1980 Mar;39(3):599–606. [PMC free article] [PubMed] [Google Scholar]
- Sayers T. J., Binder T., Berg P. A. Heterogeneity of anti-mitochondrial antibodies: characterization and separation of the antigen associated with the pseudolupus erythematosus syndrome. Clin Exp Immunol. 1979 Jul;37(1):68–75. [PMC free article] [PubMed] [Google Scholar]
- Sayers T., Leoutsakos A., Berg P., Baum H. Antimitochondrial antibodies (AMA) in primary biliary cirrhosis. I. Separation of the PBC antigen activity from mitochondrial ATPase activity. J Bioenerg Biomembr. 1981 Dec;13(5-6):255–267. doi: 10.1007/BF00743204. [DOI] [PubMed] [Google Scholar]
- Tuena De Gómez-Puyou M., Gómez-Puyou A. A simple method of purification of a soluble oligomycin-insensitive mitochondrial ATPase. Arch Biochem Biophys. 1977 Jul;182(1):82–86. doi: 10.1016/0003-9861(77)90285-5. [DOI] [PubMed] [Google Scholar]