Abstract
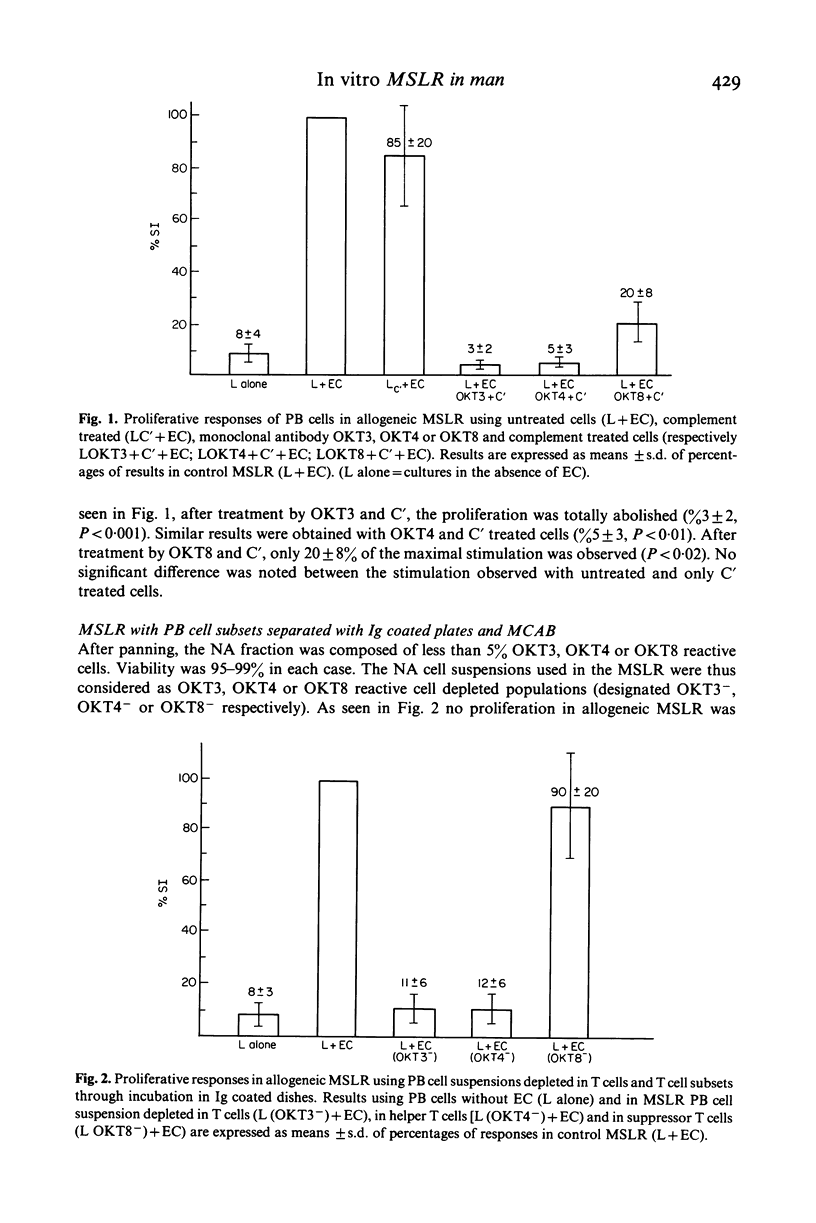
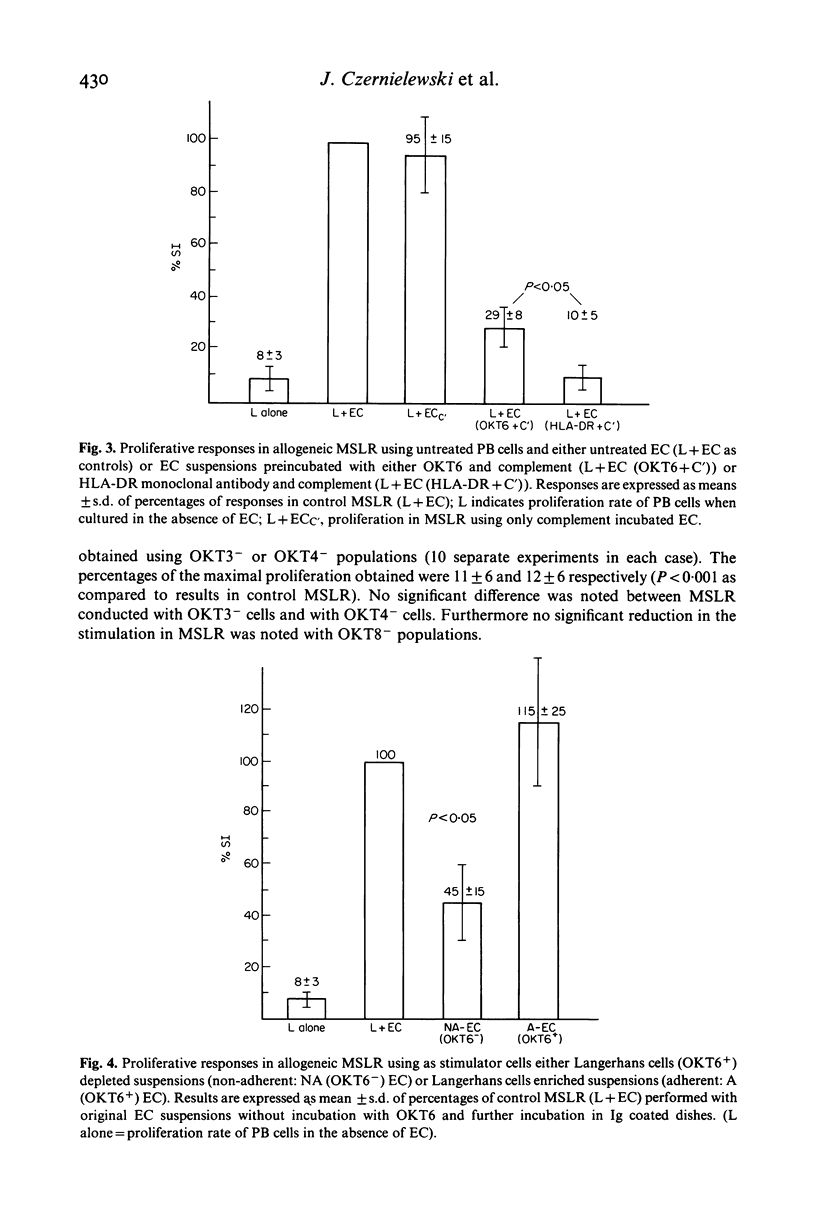
The nature of normal human epidermal cells (EC) and peripheral blood (PB) cells that react in vitro in allogeneic mixed skin cell lymphocyte culture reaction (MSLR) was investigated using monoclonal antibodies (MCAB) specific for cell subpopulations. T cells and helper-inducer and suppressor-cytotoxic T cell subsets were defined by OKT3, OKT4, OKT8 MCAB, respectively, whereas, among EC, Langerhans cells were characterized by reactivity with OKT6 or anti-HLA-DR MCAB. MSLR were conducted with untreated cell suspensions as controls and cells suspensions depleted of various functionally active cell subset(s). Two approaches were used for cell depletion: (1) complement (C')-mediated lysis by MCAB of T cells, T cell subsets, HLA-DR or OKT6 positive cells; (2) panning of PB cells or EC after pre-incubation with the appropriate MCAB to deplete or enrich (OKT6) cell suspensions with the respective cell subset. Responses in MSLR were abolished after treatment of PB cells with OKT3 + C' or OKT4 + C', significantly reduced with OKT8 + C'; they were abolished after incubation of EC with anti-HLA-DR + C' and significantly reduced with OKT6 + C'. After panning, OKT3 and OKT4 depleted populations did not proliferate in MSLR while OKT8 depleted populations respond as controls. OKT6 depleted EC were not able to stimulate PB cells, yet proliferation rates were increased after stimulation by OKT6 enriched EC. Data show that helper-inducer T cells (OKT3+; OKT4+) play the major role in MSLR and that the presence of Langerhans cells is necessary for the stimulation of PB cells. They also suggest that co-operation between helper and suppressor cells is necessary for an optimal response. Differences in results using either OKT6 or anti-HLA-Dr-C'-mediated treatment of EC may be related to differences in the cellular expression of these markers by EC.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beucher F., Saurat J. H. Stimulation des lymphocytes du sang périphérique chez le Cobaye par des cellules épidermiques autologues. C R Seances Acad Sci D. 1979 Nov 26;289(14):1041–1044. [PubMed] [Google Scholar]
- Braathen L. R., Thorsby E. Studies on human epidermal Langerhans cells. I. Allo-activating and antigen-presenting capacity. Scand J Immunol. 1980;11(4):401–408. doi: 10.1111/j.1365-3083.1980.tb00006.x. [DOI] [PubMed] [Google Scholar]
- Bystryn J. C. Epidermal antigens. Int J Dermatol. 1977 Oct;16(8):645–656. doi: 10.1111/j.1365-4362.1977.tb01875.x. [DOI] [PubMed] [Google Scholar]
- Damle N. K., Hansen J. A., Good R. A., Gupta S. Monoclonal antibody analysis of human T lymphocyte subpopulations exhibiting autologous mixed lymphocyte reaction. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5096–5098. doi: 10.1073/pnas.78.8.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisinger M., Lee J. S., Hefton J. M., Darzynkiewicz Z., Chiao J. W., de Harven E. Human epidermal cell cultures: growth and differentiation in the absence of differentiation in the absence of dermal components or medium supplements. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5340–5344. doi: 10.1073/pnas.76.10.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman E. G., Benike C. J., Grumet F. C., Evans R. L. Activation of human T lymphocyte subsets: helper and suppressor/cytotoxic T cells recognize and respond to distinct histocompatibility antigens. J Immunol. 1981 Nov;127(5):2124–2129. [PubMed] [Google Scholar]
- Faure M., Eisinger M., Bystryn J. C. Pemphigus, pemphigoid, and epidermal upper-cytoplasmic antigens: changes in expression in cultured human keratinocytes. Arch Dermatol Res. 1981;271(1):73–82. doi: 10.1007/BF00417390. [DOI] [PubMed] [Google Scholar]
- Fithian E., Kung P., Goldstein G., Rubenfeld M., Fenoglio C., Edelson R. Reactivity of Langerhans cells with hybridoma antibody. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2541–2544. doi: 10.1073/pnas.78.4.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg H., Thorsby E. Lymphocyte activating alloantigens on human epidermal cells. Tissue Antigens. 1975 Oct;6(4):183–194. doi: 10.1111/j.1399-0039.1975.tb00633.x. [DOI] [PubMed] [Google Scholar]
- Klareskog L., Tjernlund U., Forsum U., Peterson P. A. Epidermal Langerhans cells express Ia antigens. Nature. 1977 Jul 21;268(5617):248–250. doi: 10.1038/268248a0. [DOI] [PubMed] [Google Scholar]
- Lampert I. A., Suitters A. J., Chisholm P. M. Expression of Ia antigen on epidermal keratinocytes in graft-versus-host disease. Nature. 1981 Sep 10;293(5828):149–150. doi: 10.1038/293149a0. [DOI] [PubMed] [Google Scholar]
- Lane J. T., Jackson L., Ling N. R. Lymphocyte stimulation with skin cells. A function of maturation. Transplantation. 1975 Mar;19(3):250–259. doi: 10.1097/00007890-197503000-00009. [DOI] [PubMed] [Google Scholar]
- Mason D. W., Dallman M., Barclay A. N. Graft-versus-host disease induces expression of Ia antigen in rat epidermal cells and gut epithelium. Nature. 1981 Sep 10;293(5828):150–151. doi: 10.1038/293150a0. [DOI] [PubMed] [Google Scholar]
- Murphy G. F., Bhan A. K., Sato S., Mihm M. C., Jr, Harrist T. J. A new immunologic marker for human Langerhans cells. N Engl J Med. 1981 Mar 26;304(13):791–792. doi: 10.1056/NEJM198103263041320. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with the human cytotoxic/suppressor T cell subset previously defined by a heteroantiserum termed TH2. J Immunol. 1980 Mar;124(3):1301–1307. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Further characterization of the human inducer T cell subset defined by monoclonal antibody. J Immunol. 1979 Dec;123(6):2894–2896. [PubMed] [Google Scholar]
- Reinherz E. L., Penta A. C., Hussey R. E., Schlossman S. F. A rapid method for separating functionally intact human T lymphocytes with monoclonal antibodies. Clin Immunol Immunopathol. 1981 Nov;21(2):257–266. doi: 10.1016/0090-1229(81)90214-2. [DOI] [PubMed] [Google Scholar]
- Rowden G., Lewis M. G., Sullivan A. K. Ia antigen expression on human epidermal Langerhans cells. Nature. 1977 Jul 21;268(5617):247–248. doi: 10.1038/268247a0. [DOI] [PubMed] [Google Scholar]
- Stingl G., Katz S. I., Abelson L. D., Mann D. L. Immunofluorescent detection of human B cell alloantigens on S-Ig-positive lymphocytes and epidermal Langerhans cells. J Immunol. 1978 Feb;120(2):661–664. [PubMed] [Google Scholar]
- Stingl G., Katz S. I., Shevach E. M., Wolff-Schreiner E., Green I. Detection of Ia antigens on Langerhans cells in guinea pig skin. J Immunol. 1978 Feb;120(2):570–578. [PubMed] [Google Scholar]
- Tanaka S., Sakai A. Stimulation of allogeneic lymphocytes by skin epidermal cells in the rat. Transplantation. 1979 Mar;27(3):194–199. doi: 10.1097/00007890-197903000-00011. [DOI] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]


