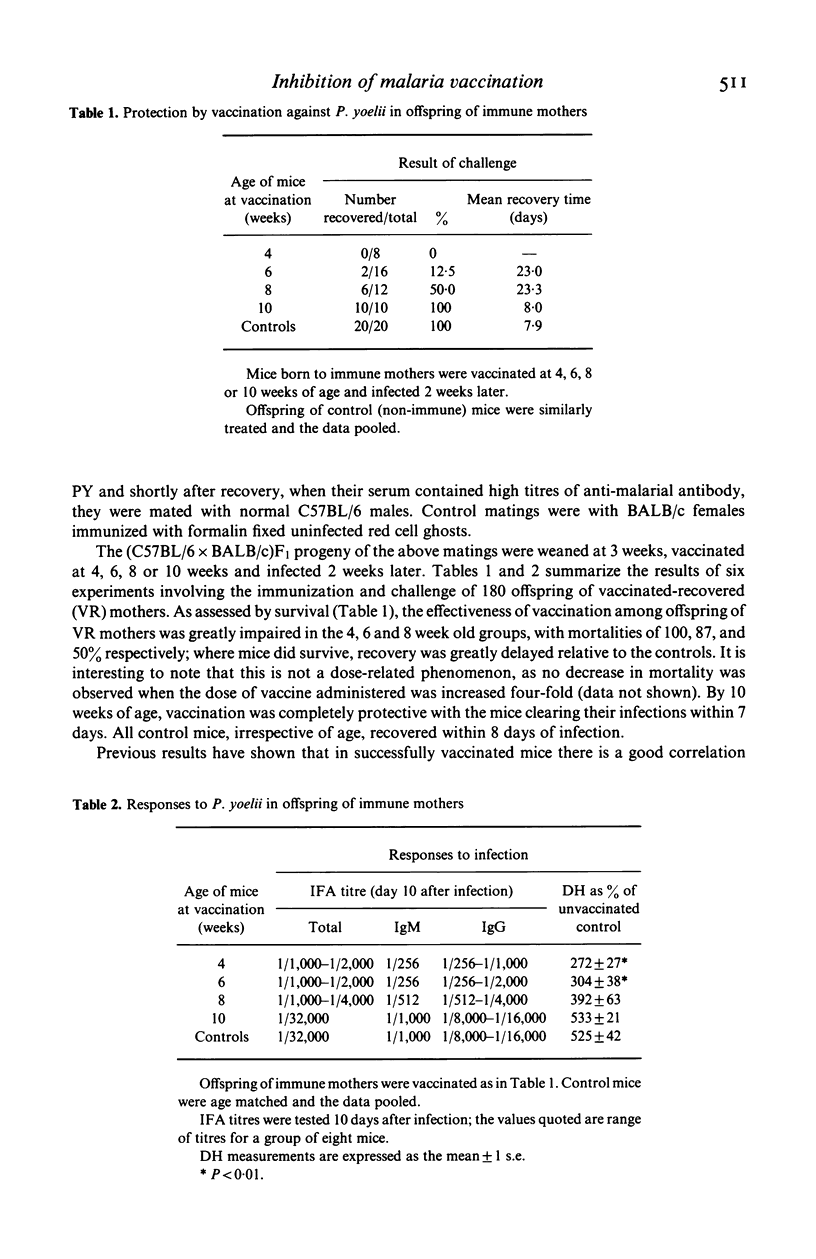
Abstract
Female BALB/c mice were vaccinated against blood stage P. yoelii (17XL strain), infected 2 weeks later and after recovery mated to normal C57B1/6 males. Control matings were with normal BALB/c females. The (C57B1/6 x BALB/c)F1 progeny were vaccinated at 4, 6, 8 or 10 weeks of age and infected 2 weeks later with lethal P. yoelii. All control mice were fully protected, but in the offspring of immune mothers mortality was 100, 87, 50, and 0% respectively. Mice in which the protective effect of vaccination had been abolished showed greatly reduced specific IgG and delayed hypersensitivity (DH) responses to challenge with parasite antigen. Results indicate that this failure of vaccination is due to the transmission of maternal IgG to the offspring which acts to suppress both priming by the vaccine and the generation of specific T helper cells involved in IgG production, as measured by the response to TNP-P. yoelii.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARR M., GLENNY A. T., RANDALL K. J. Diphtheria immunization in young babies; a study of some factors involved. Lancet. 1950 Jan 7;1(6593):6–10. doi: 10.1016/s0140-6736(50)90213-3. [DOI] [PubMed] [Google Scholar]
- BRUCE-CHWATT L. J., GIBSON F. D. Transplacental passage of Plasmodium berghei and passive transfer of immunity in rats and mice. Trans R Soc Trop Med Hyg. 1956 Jan;50(1):47–53. doi: 10.1016/0035-9203(56)90007-4. [DOI] [PubMed] [Google Scholar]
- Cohen S. Immunity to malaria. Proc R Soc Lond B Biol Sci. 1979 Jan 15;203(1153):323–345. doi: 10.1098/rspb.1979.0001. [DOI] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Desowitz R. S. Some factors influencing the induction, maintenance and degree of maternally transmitted protective immunity to malaria (Plasmodium berghei). Trans R Soc Trop Med Hyg. 1973;67(2):238–244. doi: 10.1016/0035-9203(73)90150-8. [DOI] [PubMed] [Google Scholar]
- Dresser D. W., Popham A. M. The influence of T cells on the initiation and expression of immunological memory. Immunology. 1979 Oct;38(2):265–274. [PMC free article] [PubMed] [Google Scholar]
- Eichmann K. Idiotype suppression. II. Amplification of a suppressor T cell with anti-idiotypic activity. Eur J Immunol. 1975 Aug;5(8):511–517. doi: 10.1002/eji.1830050802. [DOI] [PubMed] [Google Scholar]
- MCGREGOR I. A., WILLIAMS K., VOLLER A., BILLEWICZ W. Z. IMMUNOFLUORESCENCE AND THE MEASUREMENT OF IMMUNE RESPONSE TO HYPERENDEMIC MALARIA. Trans R Soc Trop Med Hyg. 1965 Jul;59:395–414. doi: 10.1016/0035-9203(65)90057-x. [DOI] [PubMed] [Google Scholar]
- MOLLER G., WIGZELL H. ANTIBODY SYNTHESIS AT THE CELLULAR LEVEL. ANTIBODY-INDUCED SUPPRESSION OF 19S AND 7S ANTIBODY RESPONSE. J Exp Med. 1965 Jun 1;121:969–989. doi: 10.1084/jem.121.6.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molineaux L., Cornille-Brögger R., Mathews H. M., Storey J. Longitudinal serological study of malaria in infants in the West African savanna. Comparisons in infants exposed to, or protected from, transmission from birth. Bull World Health Organ. 1978;56(4):573–578. [PMC free article] [PubMed] [Google Scholar]
- Orjih A. U., Cochrane A. H., Nussenzweig R. S. Active immunization and passive transfer of resistance against sporozoite-induced malaria in infant mice. Nature. 1981 May 28;291(5813):331–332. doi: 10.1038/291331a0. [DOI] [PubMed] [Google Scholar]
- PERKINS F. T., YETTS R., GAISFORD W. Response of infants to a third dose of poliomyelitis vaccine given 10 to 12 months after primary immunization. Br Med J. 1959 Mar 14;1(5123):680–682. doi: 10.1136/bmj.1.5123.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer T. T. Plasmodium berghei infection in pregnant rats: effects on antibody response and course of infection in offspring. J Parasitol. 1978 Jun;64(3):493–496. [PubMed] [Google Scholar]
- Playfair J. H., De Souza J. B. Antibody responses in mice protected against malaria by vaccination. Parasite Immunol. 1979 Autumn;1(3):197–208. doi: 10.1111/j.1365-3024.1979.tb00706.x. [DOI] [PubMed] [Google Scholar]
- Playfair J. H., De Souza J. B., Cottrell B. J. Reactivity and crossreactivity of mouse helper T cells to malaria parasites. Immunology. 1977 May;32(5):681–687. [PMC free article] [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- UHR J. W., BAUMANN J. B. Antibody formation. I. The suppression of antibody formation by passively administered antibody. J Exp Med. 1961 May 1;113:935–957. doi: 10.1084/jem.113.5.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voller A., O'Neill P. Immunofluorescence method suitable for large-scale application to malaria. Bull World Health Organ. 1971;45(4):524–529. [PMC free article] [PubMed] [Google Scholar]


