Abstract
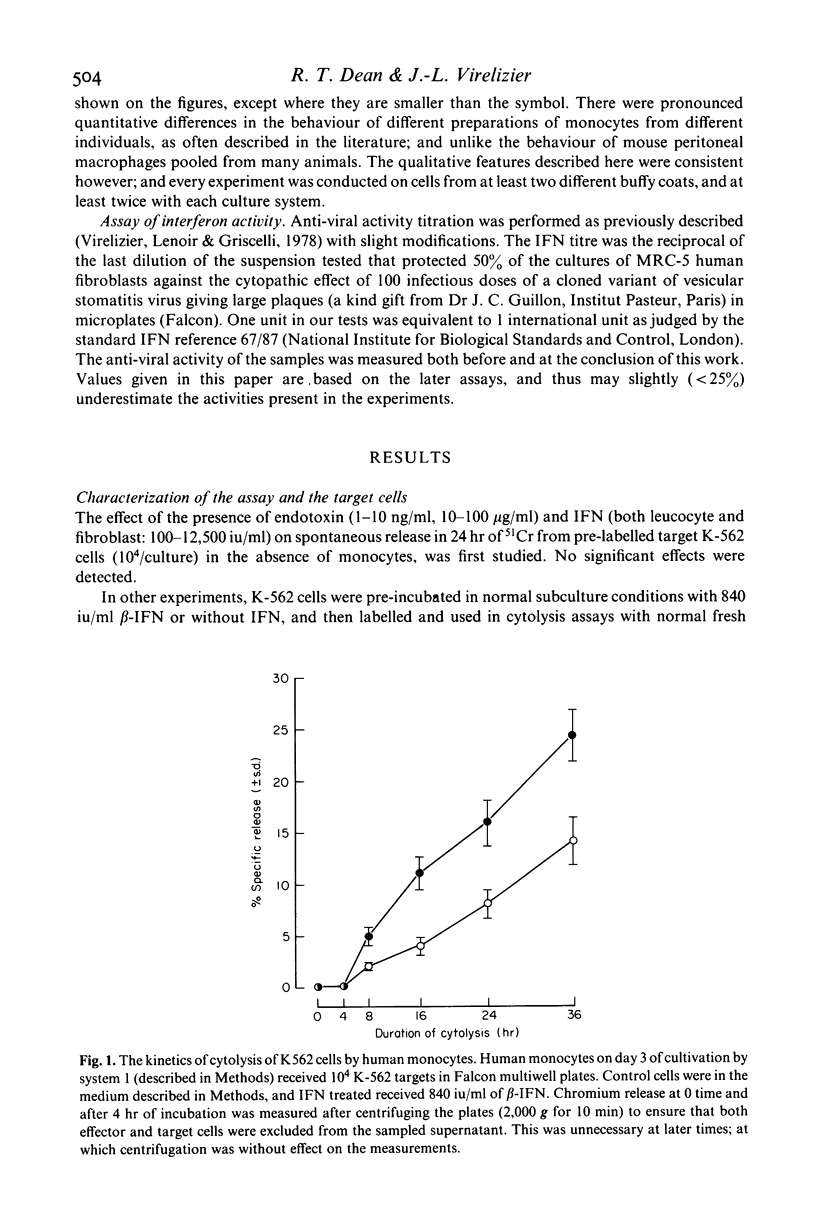
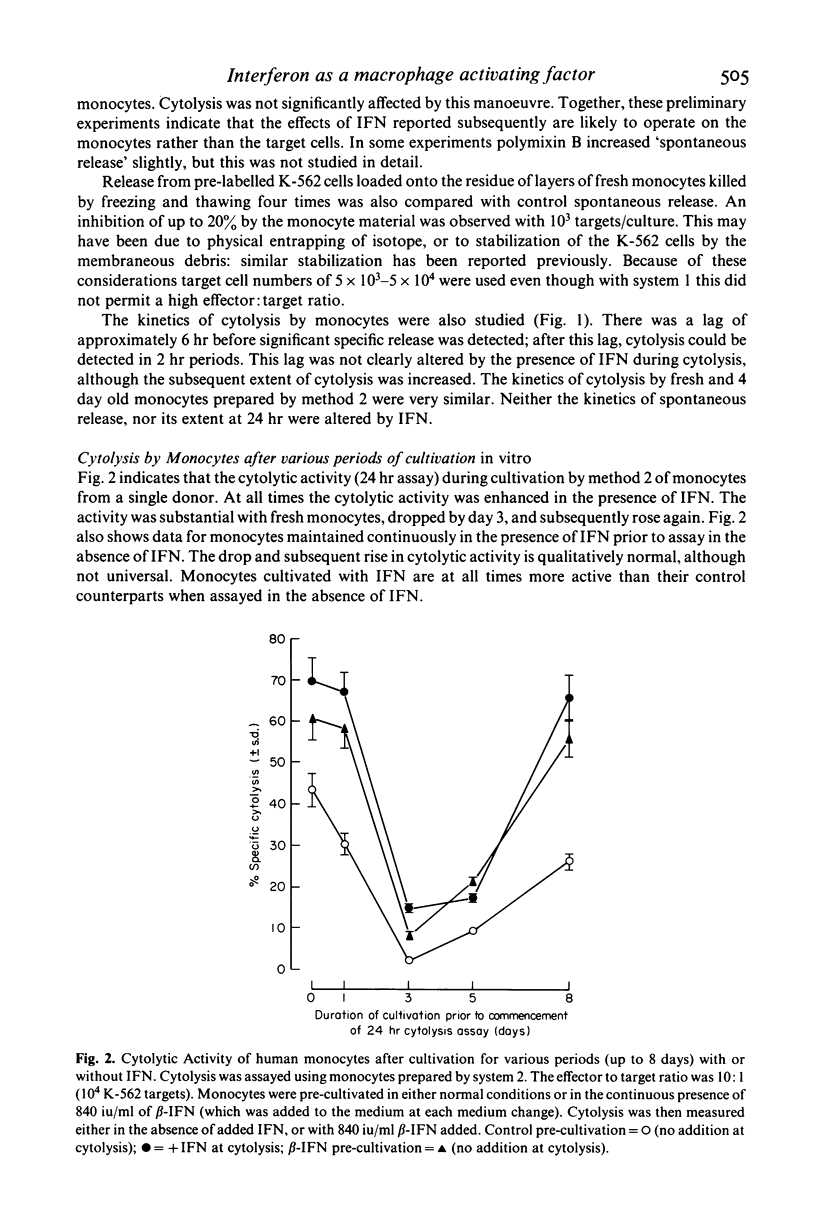
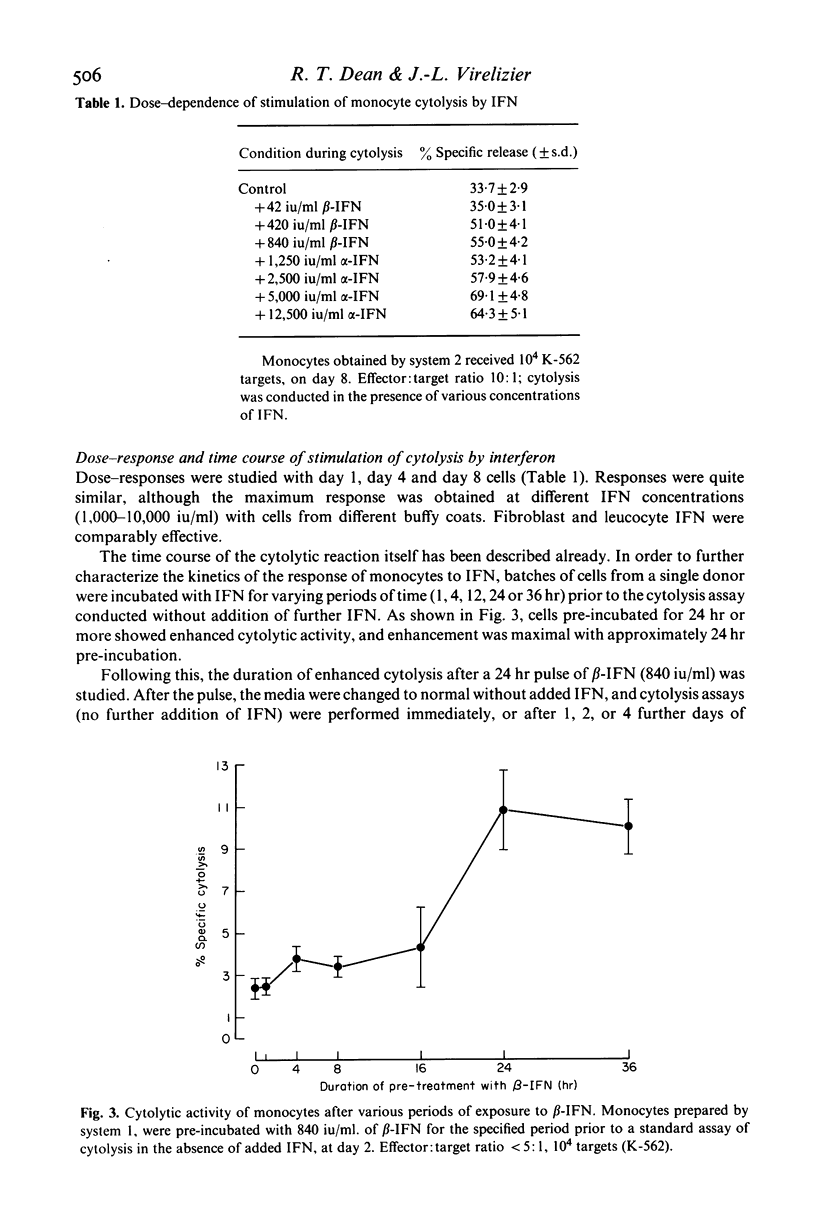
The cytolytic activity of human peripheral blood monocytes in vitro against K-562 human leukaemic target cells was stimulated by human fibroblast (beta-) and leucocyte (alpha-) interferon (IFN). Stimulation was by up to several times the corresponding control activity, and was observed with freshly isolated monocytes, and with monocytes cultured for various periods up to 10 days. The cytolytic activity of untreated monocytes was detectable at very low effector: target ratios (less than 5:1), and fell between days 1 and 4 in culture, normally rising again towards the initial activity at day 8; this pattern was also observed when IFN was present continuously, although the activities were then always higher than in the corresponding control cells. Cytolysis showed a lag of about 6 hr, in contrast to that by natural killer (NK) cells, and was routinely measured over 24 hr. The course of stimulation by IFN and its dose-response were studied. Stimulation required the presence of IFN for at least 24 hr, and was maximal with between 1,000 and 10,000 units of IFN/ml. When IFN containing media were removed and replaced with control media, the monocyte activity remained stimulated for at least 4 days. Stimulation by beta-IFN was blocked by a specific antibody to beta-IFN, under conditions in which assayable IFN activity was also neutralized. Several control experiments indicated that the action of IFN was on the monocytes and not on the target cells. The morphological maturation of monocytes was retarded by IFN, even in cultures containing up to 50% serum. The effectiveness of fibroblast IFN indicated that stimulation could not be attributed to the lymphokines which might contaminate alpha-IFN. The action of IFN did not require simultaneous or antecedent in vitro stimulation by endotoxin. This was indicated both by serum free experiments, and also by others in which polymixin B was used to complex with and render unavailable any endotoxin present. Endotoxin showed an independent stimulatory effect, which could be prevented by polymixin.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom B. R. Interferons and the immune system. Nature. 1980 Apr 17;284(5757):593–595. doi: 10.1038/284593a0. [DOI] [PubMed] [Google Scholar]
- Boraschi D., Tagliabue A. Interferon-induced enhancement of macrophage-mediated tumor cytolysis and its difference from activation by lymphokines. Eur J Immunol. 1981 Feb;11(2):110–114. doi: 10.1002/eji.1830110209. [DOI] [PubMed] [Google Scholar]
- Dean R. T., Hylton W., Allison A. C. Induction of macrophage lysosomal enzyme secretion by agents acting at the plasma membrane. Exp Cell Biol. 1979;47(6):454–462. doi: 10.1159/000162963. [DOI] [PubMed] [Google Scholar]
- Fischer D. G., Hubbard W. J., Koren H. S. Tumor cell killing by freshly isolated peripheral blood monocytes. Cell Immunol. 1981 Mar 1;58(2):426–435. doi: 10.1016/0008-8749(81)90235-5. [DOI] [PubMed] [Google Scholar]
- Gerrard T. L., Terz J. J., Kaplan A. M. Cytotoxicity to tumor cells of monocytes from normal individuals and cancer patients. Int J Cancer. 1980 Nov 15;26(5):585–593. doi: 10.1002/ijc.2910260510. [DOI] [PubMed] [Google Scholar]
- Hammerstrøm J. Human adherent mononuclear blood cells: cytolytic and cytostatic activity and characterization of effector cells during in vitro culture. Acta Pathol Microbiol Scand C. 1981 Apr;89(2):115–122. doi: 10.1111/j.1699-0463.1981.tb02674.x. [DOI] [PubMed] [Google Scholar]
- Hammerstrøm J. Human monocyte-mediated cytotoxicity to K-562 cells: activation by lymphokines. Scand J Immunol. 1979;10(6):575–584. doi: 10.1111/j.1365-3083.1979.tb01392.x. [DOI] [PubMed] [Google Scholar]
- Hammerstrøm J. In vitro influence of endotoxin on human mononuclear phagocyte structure and function. 2. Enhancement of the expression of cytoststic and cytolytic activity of normal and lymphokine-activated monocytes. Acta Pathol Microbiol Scand C. 1979 Dec;87(6):391–399. [PubMed] [Google Scholar]
- Horwitz D. A., Kight N., Temple A., Allison A. C. Spontaneous and induced cytotoxic properties of human adherent mononuclear cells: killing of non-sensitized and antibody-coated non-erythroid cells. Immunology. 1979 Feb;36(2):221–228. [PMC free article] [PubMed] [Google Scholar]
- Jett J. R., Mantovani A., Herberman R. B. Augmentation of human monocyte-mediated cytolysis by interferon. Cell Immunol. 1980 Sep 1;54(2):425–434. doi: 10.1016/0008-8749(80)90222-1. [DOI] [PubMed] [Google Scholar]
- Johnson W. D., Jr, Mei B., Cohn Z. A. The separation, long-term cultivation, and maturation of the human monocyte. J Exp Med. 1977 Dec 1;146(6):1613–1626. doi: 10.1084/jem.146.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. H., Epstein L. B. Reversible inhibition by interferon of the maturation of human peripheral blood monocytes to macrophages. Cell Immunol. 1980 Mar 1;50(1):177–190. doi: 10.1016/0008-8749(80)90016-7. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Brukner L. H., Silverstein S. C., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. I. Pharmacologic triggering of effector cells and the release of hydrogen peroxide. J Exp Med. 1979 Jan 1;149(1):84–99. doi: 10.1084/jem.149.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R. M., Chirigos M. A., Heine U. I. Functional and morphologic characteristics of interferon-treated macrophages. Cell Immunol. 1978 Jan;35(1):84–91. doi: 10.1016/0008-8749(78)90128-4. [DOI] [PubMed] [Google Scholar]
- Stanwick T. L., Campbell D. E., Nahmias A. J. Spontaneous cytotoxicity mediated by human monocyte-macrophages against human fibroblasts infected with herpes simplex virsu--augmentation by interferon. Cell Immunol. 1980 Aug 1;53(2):413–416. doi: 10.1016/0008-8749(80)90342-1. [DOI] [PubMed] [Google Scholar]
- Taichman N. S., Dean R. T., Sanderson C. J. Biochemical and morphological characterization of the killing of human monocytes by a leukotoxin derived from Actinobacillus actinomycetemcomitans. Infect Immun. 1980 Apr;28(1):258–268. doi: 10.1128/iai.28.1.258-268.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taramelli D., Holden H. T., Varesio L. Endotoxin requirement for macrophage activation by lymphokines in a rapid microcytotoxicity assay. J Immunol Methods. 1980;37(3-4):225–232. doi: 10.1016/0022-1759(80)90309-9. [DOI] [PubMed] [Google Scholar]
- Virelizier J. L., Allison A. C., de Maeyer E. Production by mixed lymphocyte cultures of a type II interferon able to protect macrophages against virus infection. Infect Immun. 1977 Aug;17(2):282–285. doi: 10.1128/iai.17.2.282-285.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virelizier J. L., Lenoir G., Griscelli C. Persistent Epstein-Barr virus infection in a child with hypergammaglobulinaemia and immunoblastic proliferation associated with a selective defect in immune interferon secretion. Lancet. 1978 Jul 29;2(8083):231–234. doi: 10.1016/s0140-6736(78)91744-0. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Karre K., Hansson M., Kunkel L. A., Kiessling R. W. Interferon-mediated protection of normal and tumor target cells against lysis by mouse natural killer cells. J Immunol. 1981 Jan;126(1):219–225. [PubMed] [Google Scholar]


