Abstract
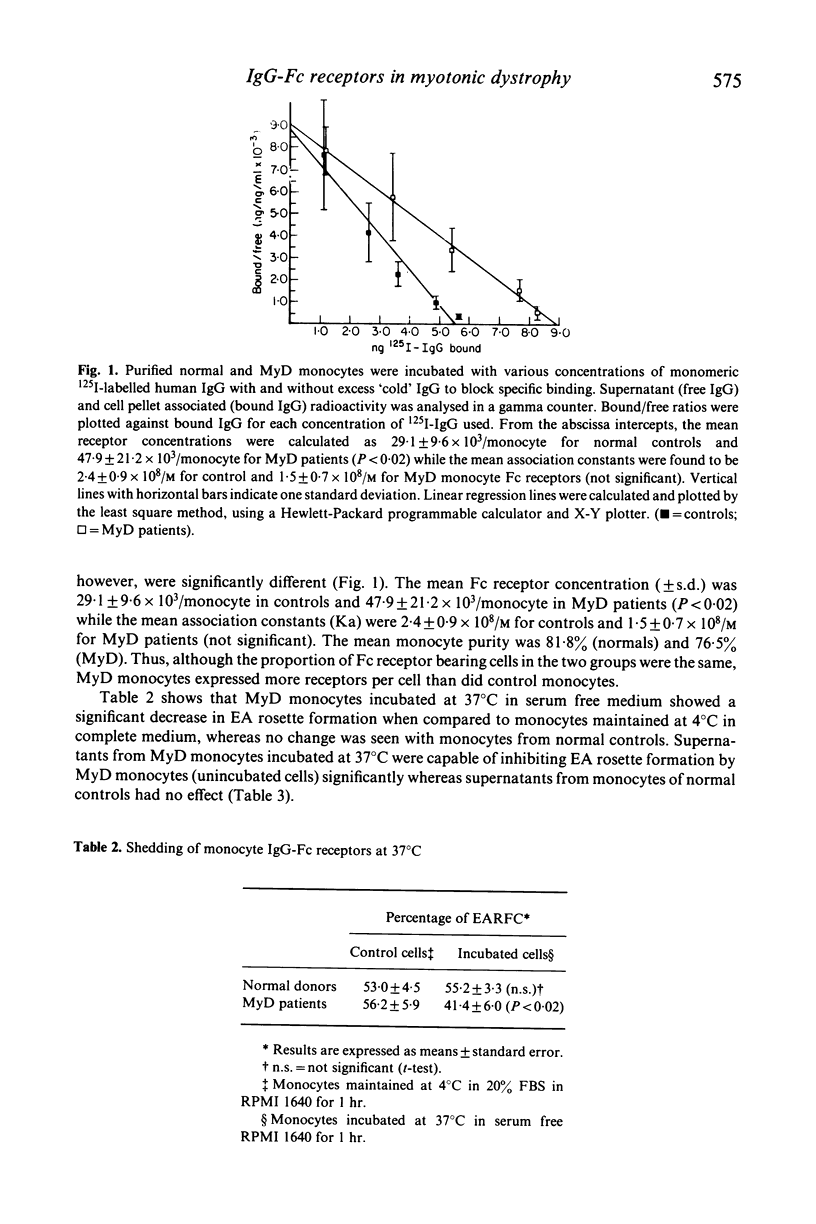
Myotonic dystrophy (MyD), an autosomal dominant neuromuscular disease with multisystem abnormalities, is associated with hypercatabolism of IgG. The hypercatabolism is not related to structural abnormalities of the IgG molecule in MyD but appears to be due to a derangement of the serum IgG concentration-fractional catabolic rate relationship. Since the catabolic pattern of IgG is governed by the Fc portion of the molecule, the possibility of Fc receptor dysfunction in MyD has been explored. We have observed that although MyD patients have normal numbers of Fc receptor bearing leucocytes in their peripheral blood, MyD monocytes express significantly (P less than 0.02) greater numbers of Fc receptors (47.9 +/- 21.2 X 10(3) receptors/monocyte) than do monocytes of healthy subjects (29.1 +/- 9.6 X 10(3) receptors/monocyte). The mean affinity constants of the Fc receptors was lower in the MyD group (1.5 +/- 0.7 X 10(8)/M) than the normal control group (2.4 +/- 0.9 X 10(8)/M) but this difference was not statistically significant. MyD monocytes showed a propensity to shed Fc receptors in culture at 37 degrees C whereas no significant shedding was observed with control monocytes. Thus MyD monocytes may shed Fc receptors at physiological temperatures but at the same time express more receptors per cell than normal monocytes. This suggests that MyD monocytes may have an abnormally high turn-over of Fc receptors.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN S. B., BJORNEBOE M. GAMMA GLOBULIN TURNOVER IN RABBITS BEFORE AND DURING HYPERIMMUNIZATION. J Exp Med. 1964 Apr 1;119:537–546. doi: 10.1084/jem.119.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASKONAS B. A., HUMPHREY J. H. Formation of antibody by isolated perfused lungs of immunized rabbits: the use of [14C]amino acids to study the dynamics of antibody secretion. Biochem J. 1958 Oct;70(2):212–222. doi: 10.1042/bj0700212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENACERRAF B., SEBESTYEN M., COOPER N. S. The clearance of antigen antibody complexes from the blood by the reticuloendothelial system. J Immunol. 1959 Feb;82(2):131–137. [PubMed] [Google Scholar]
- BIRKE G., LILJEDAHL S. O., OLHAGEN B., PLANTIN L. O., AHLINDER S. Catabolism and distribution of gamma-globulin. A preliminary study with 131 I-labelled gammaglobulin. Acta Med Scand. 1963 May;173:589–603. [PubMed] [Google Scholar]
- BRAMBELL F. W., HEMMINGS W. A., MORRIS I. G. A THEORETICAL MODEL OF GAMMA-GLOBULIN CATABOLISM. Nature. 1964 Sep 26;203:1352–1354. doi: 10.1038/2031352a0. [DOI] [PubMed] [Google Scholar]
- COHEN S., GORDON A. H., MATTHEWS C. Catabolism of gamma-globulin by the isolated perfused rat liver. Biochem J. 1962 Jan;82:197–205. doi: 10.1042/bj0820197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREEMAN T., GORDON A. H., HUMPHREY J. H. Distinction between catabolism of native and denatured proteins by the isolated perfused liver after carbon loading. Br J Exp Pathol. 1958 Oct;39(5):459–471. [PMC free article] [PubMed] [Google Scholar]
- Froland S. S., Wisloff F., Michaelsen T. E. Human lymphocytes with receptors for IgG. A population of cells distinct from T- and B-lymphocytes. Int Arch Allergy Appl Immunol. 1974;47(1):124–138. doi: 10.1159/000231207. [DOI] [PubMed] [Google Scholar]
- Fukumoto T., Brandon M. R. The site of IgG2a catabolism in the rat. Mol Immunol. 1981 Aug;18(8):741–750. doi: 10.1016/0161-5890(81)90066-3. [DOI] [PubMed] [Google Scholar]
- Hay F. C., Torrigiani G., Roitt I. M. The binding of human IgG subclasses to human monocytes. Eur J Immunol. 1972 Jun;2(3):257–261. doi: 10.1002/eji.1830020312. [DOI] [PubMed] [Google Scholar]
- Jarnum S., Andersen S. B., Dich J., Hein M. F., Henriksen F. W., Schmidt A. Pancreatic catabolism of albumin and gamma-globulin. Clin Sci. 1966 Oct;31(2):223–230. [PubMed] [Google Scholar]
- Kumagai K., Itoh K., Hinuma S., Tada M. Pretreatment of plastic Petri dishes with fetal calf serum. A simple method for macrophage isolation. J Immunol Methods. 1979;29(1):17–25. doi: 10.1016/0022-1759(79)90121-2. [DOI] [PubMed] [Google Scholar]
- LIPPINCOTT S. W., KORMAN S., FONG C., STICKLEY E., WOLINS W., HUGHES W. L. Turnover of labeled normal gamma globulin in multiple myeloma. J Clin Invest. 1960 Apr;39:565–572. doi: 10.1172/JCI104069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okafor G. O., Turner M. W., Hay F. C. Localisation of monocyte binding site of human immunoglobulin G. Nature. 1974 Mar 15;248(445):228–230. doi: 10.1038/248228a0. [DOI] [PubMed] [Google Scholar]
- SOLOMON A., WALDMANN T. A., FAHEY J. L. Clinical and experimental metabolism of normal 6.6s gamma-globulin in normal subjects and in patients with macroglobulinemia and multiple myeloma. J Lab Clin Med. 1963 Jul;62:1–17. [PubMed] [Google Scholar]
- Schmidt M. E., Douglas S. D. Disappearance and recovery of human monocyte IgG receptor activity after phagocytosis. J Immunol. 1972 Oct;109(4):914–917. [PubMed] [Google Scholar]
- Sonoda S., Schlamowitz M. Studies of 125I trace labeling of immunoglobulin G by chloramine-T. Immunochemistry. 1970 Nov;7(11):885–898. doi: 10.1016/0019-2791(70)90051-0. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C., Eisen H. N. Binding of monomeric immunoglobulins to Fc receptors of mouse macrophages. J Exp Med. 1975 Dec 1;142(6):1520–1533. doi: 10.1084/jem.142.6.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A., Morell A. G., Wochner R. D., Strober W., Sternlieb I. Measurement of gastrointestinal protein loss using ceruloplasmin labeled with copper. J Clin Invest. 1967 Jan;46(1):10–20. doi: 10.1172/JCI105502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A., Strober W. Metabolism of immunoglobulins. Prog Allergy. 1969;13:1–110. doi: 10.1159/000385919. [DOI] [PubMed] [Google Scholar]
- Wochner R. D., Drews G., Strober W., Waldmann T. A. Accelerated breakdown of immunoglobulin G (IgG) in myotonic dystrophy: a hereditary error of immunoglobulin catabolism. J Clin Invest. 1966 Mar;45(3):321–329. doi: 10.1172/JCI105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wochner R. D., Strober W., Waldmann T. A. The role of the kidney in the catabolism of Bence Jones proteins and immunoglobulin fragments. J Exp Med. 1967 Aug 1;126(2):207–221. doi: 10.1084/jem.126.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]


