Abstract
FOR INUIT CHILDREN, A TRADITIONAL DIET contains 20 mg of elemental calcium per day, well below the recommended daily intake. To identify alterations in intestinal or renal calcium absorption, 10 healthy Inuit children (5 to 17 years of age) were given a standardized calcium load (Pak test). Five had hypercalciuria (hyperabsorptive in 3 and renal leak in 2), a frequency markedly different from that for white children (p < 0.004) and not explained by calcitropic hormone and serum calcium levels, which were normal. There was a preponderance of the bb vitamin D receptor genotype (8 of 10 subjects; p < 0.01 for comparison with white populations). Dietary calcium absorption appeared to be more efficient in these Inuit children, with an increased frequency of hypercalciuria associated with the bb genotype. This may represent a genetic adaptation to dietary constraints and may predispose to nephrolithiasis or nephrocalcinosis if standard nutritional guidelines are followed.
Recommended elemental calcium intakes for North American children are 800 mg/d for those 4 to 8 years old and 1300 mg/d for those 9 years of age and older.1 With a traditional diet, Inuit children in northern Canada ingest only 20 mg of elemental calcium per day.2 After presumably adapting to this constraint over millennia, the Inuit are now adopting a more southern “market” diet, with appreciably higher calcium intakes. Having observed cases of severe hypercalciuria and nephrocalcinosis in children from northern communities, we hypothesized that adaptation to a restricted calcium intake might be associated with altered intestinal or renal tubular calcium absorption, which might be maladaptive as the traditional diet is supplanted. In this situation, increased dietary calcium absorption might lead to hypercalciuria, nephrocalcinosis or nephrolithiasis. We used a standardized oral calcium challenge to examine intestinal calcium absorption and renal calcium handling in healthy Inuit children.
Six female and 4 male Inuit children, 5 to 17 years of age, were recruited at random from clinics of the Montreal Children's Hospital. These otherwise healthy children had come from remote Northern Quebec communities for follow-up after appendectomy (n = 4), cutaneous infection (n = 2), tonsillectomy (n = 1) and healed fracture (n = 2) and for evaluation of mild developmental delay (n = 1). The children, their parents and the treating physicians were approached at the Northern Children's Clinic to obtain informed consent; the services of a translator were used when required.
Each child underwent a pediatric Pak test with standardized oral calcium load. Urine was collected before and after a standard calcium meal, and blood was drawn for determination of vitamin D receptor genotype, serum calcium level and levels of calcitropic hormones. One patient (subject 2) did not receive the full calcium load and was excluded from the associated analyses.
Urinary calcium excretion of less than 0.56 μmol/mol creatinine before loading and less than 0.76 μmol/mol creatinine after loading is considered normal. A normal fasting urinary calcium:creatinine ratio combined with post-load urinary calcium excretion greater than 0.76 μmol/mol creatinine is consistent with absorptive hypercalciuria, indicating increased intestinal calcium absorption. Elevated pre- and post-load urinary calcium:creatinine ratios identify patients with so-called renal leak hypercalciuria, although the 2 forms of hypercalciuria may not be distinct etiologically.3
The pediatric Pak test has been well validated in multiple studies examining the frequency of hypercalciuria in North American and European children.4,5 In North American children, absorptive hypercalciuria occurs in 2.0% and renal leak in 4.1% (n = 48);4 in European children these conditions occur in 1.20% and 0.83% respectively (n = 236).5 In general, urinary calcium:creatinine ratios above 0.56 to 0.76 μmol/mol are considered potentially injurious and are associated with risk of renal complications, such as nephrocalcinosis, stones and tubular dysfunction.
Serum and urine calcium and creatinine were measured with a Vitros 950 analyzer (Ortho-Clinical Diagnostics, Rochester, NY). Hormone assays included 25-hydroxy vitamin D (25(OH)D), 1,25-dihydroxy vitamin D (1,25(OH)2D) (both by radioimmunoassay, Dia Sorin, Stillwater, Minn.) and intact parathyroid hormone (by chemiluminescence assay, Nicols Institute, San Juan Capistrano, Calif.). Restriction fragment length polymorphisms for the vitamin D receptor gene at the BsmI site were analyzed by polymerase chain reaction as previously described, with B designating the absence and b the presence of this site.6 Norms for white populations are bb 33%, Bb 49.6%, and BB 17% (n= 572).6,7
Fisher's exact test for proportions was used to compare calcium absorption and the BsmI polymorphism with published standards.
Approval for this project was obtained from the Research Ethics Board of the Montreal Children's Hospital.
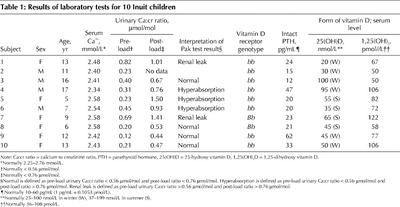
Fasting and post-load urinary calcium:creatinine ratios are shown in Table 1. Hyperabsorptive hypercalciuria was observed in 3 of 9 children (subjects 4, 5 and 6); 2 of 10 children (subjects 1 and 7) manifested renal-leak hypercalciuria before calcium loading. In 4 of 9 children, the pattern was normal. These results differ significantly from published norms for white and black children4,5 (Fisher's exact test, p < 0.004).
Table 1
Intact parathyroid hormone, serum calcium and vitamin D levels were normal, with 2 exceptions (Table 1): subject 9 had slightly higher than normal intact parathyroid hormone and subject 7 had higher than normal 1,25(OH)2D. In addition, 2 subjects (1 and 6) had borderline low levels of 25(OH)D.
The study participants had a preponderance of the bb genotype for the vitamin D receptor gene (8 children had this genotype and 2 had the Bb genotype; p < 0.01 compared with North American norms).6,7
Compared with reference populations,4,5,8 hypercalciuria was significantly more common among study participants, and observed urine calcium levels were highly elevated. Our results support both more efficient calcium absorption and increased renal losses, effects that were not explained by the normal levels of intact parathyroid hormone and 1,25(OH)2D. The distribution of vitamin D receptor genotypes was significantly different from that in the white population (p < 0.01) but was similar to that of some Asian populations. In other groups with low calcium intakes (e.g., Chinese and Thai people), bb is also the predominant genotype.9,10 This genotype is believed to be adaptive because of its association with more efficient intestinal calcium absorption. In general, these Asian populations did not demonstrate hypercalciuria with their usual diets,11 and it appears that they were able to mineralize their bones and maintain eucalcemia with a significantly lower calcium intake than recommended for the standard North American diet.
While limited by both the cross-sectional nature of this study and the small sample size, our data are nonetheless consistent with a genetic adaptation to a traditionally low-calcium diet, whereby the bb genotype appears to be associated with a high rate of hypercalciuria. Dietary calcium intakes based on North American guidelines may therefore result in iatrogenic hypercalciuria and renal damage. A cautious approach to implementing such guidelines, with recognition of genetically distinct target populations, is thus warranted. Given the dangers, these findings should also motivate more extensive longitudinal evaluation.
Acknowledgments
This project was supported by a grant from the Montreal Children's Hospital Research Foundation. Dr. Sellers was supported by a Medical Research Council of Canada Fellowship Award.
Footnotes
This article has been peer reviewed.
Contributors: Drs. Sellers, Sharma and Rodd shared equally in the research project and preparation of the manuscript. Specifically, Drs. Rodd and Sharma designed the project and assisted with the several drafts of the manuscript. Dr. Sellers acquired and analyzed most of the data and assisted with all drafts.
Competing interests: None declared.
Correspondence to: Celia Rodd, Montreal Children's Hospital, E-316, 2300 Tupper St., Montréal QC H3H 1P3; fax 514 412 4494; celia.rodd@mcgill.ca
References
- 1.Dietary reference intake for calcium, phosphorus, magnesium, vitamin D and fluoride. Washington: National Academy Press; 1999. [PubMed]
- 2.Kuhnlein H, Soueida R, Receveur O. Dietary nutrient profiles of Canadian Baffin Island Inuit differ by food source, season, and age. J Am Diet Assoc 1996; 96:155-62. [DOI] [PubMed]
- 3.Stapleton F, Jones D, Miller L. Evaluation of bone metabolism in children with hypercalciuria. Semin Nephrol 1989;9:75-8. [PubMed]
- 4.Stapleton B, Noe N, Jerkins G, Roy S 3rd. Urinary excretion of calcium following an oral calcium loading test in healthy children. Pediatrics 1982; 69: 594-7. [PubMed]
- 5.Kruse K, Kracht U, Kruse U. Reference values for urinary calcium excretion and screening for hypercalciuria in children and adolescents. Eur J Pediatr 1984;143:25-31. [DOI] [PubMed]
- 6.Kiel DP, Myers RH, Cupples LA, Kong XF, Zhu XH, Ordovas J, et al. The BsmI vitamin D receptor restriction fragment length polymorphism (bb) influences the effect of calcium intake on bone mineral density. J Bone Miner Res 1997;12:1049-57. [DOI] [PubMed]
- 7.Morrison N, Qi J, Tokita A, Kelly P, Crofts L, Nguyen T, et al. Prediction of bone density from vitamin D receptor alleles. Nature 1994;367:284-7. [DOI] [PubMed]
- 8.Moore E, Coe F, McMann B, Favus M. Idiopathic hypercalciuria in children: prevalence and metabolic characteristics. J Pediatr 1978;92:906-10. [DOI] [PubMed]
- 9.Beaven S, Prentice A, Yan L, Dibba B, Ralston S. Differences in vitamin D receptor genotype and geographical variation in osteoporosis. Lancet 1996;348:136-7. [DOI] [PubMed]
- 10.Ongphiphadhanakul B, Rajatanavin R, Chanprasertyothin S, Chailurkit L, Piaseu N, Teerarungsikul K, et al. Vitamin D receptor gene polymorphism is associated with urinary calcium excretion but not with bone mineral density in post menopausal women. J Endocrinol Invest 1997;20:592-6. [DOI] [PubMed]
- 11.Wong G, Lam C, Kwok M, Mak T. Urinary calcium excretion in Chinese adolescents. J Paediatr Child Health 1998;34:226-8. [DOI] [PubMed]