Abstract
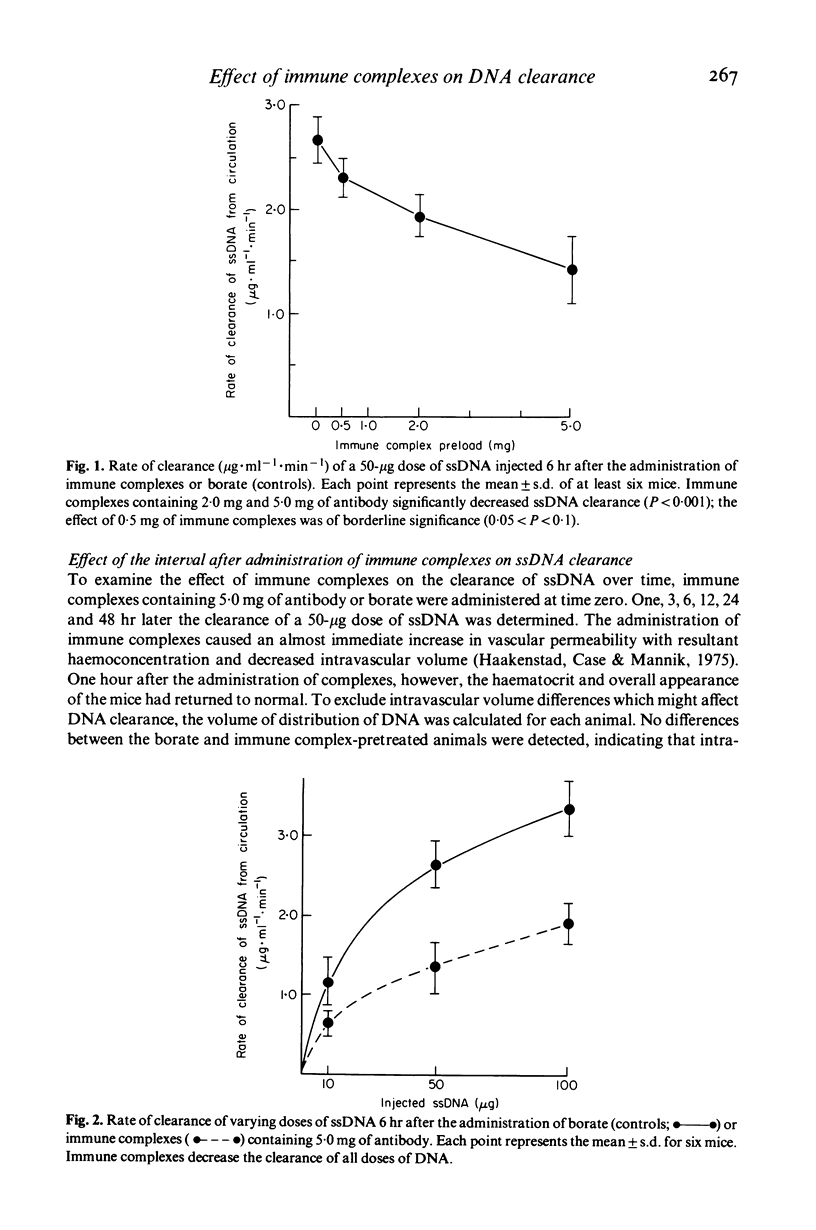
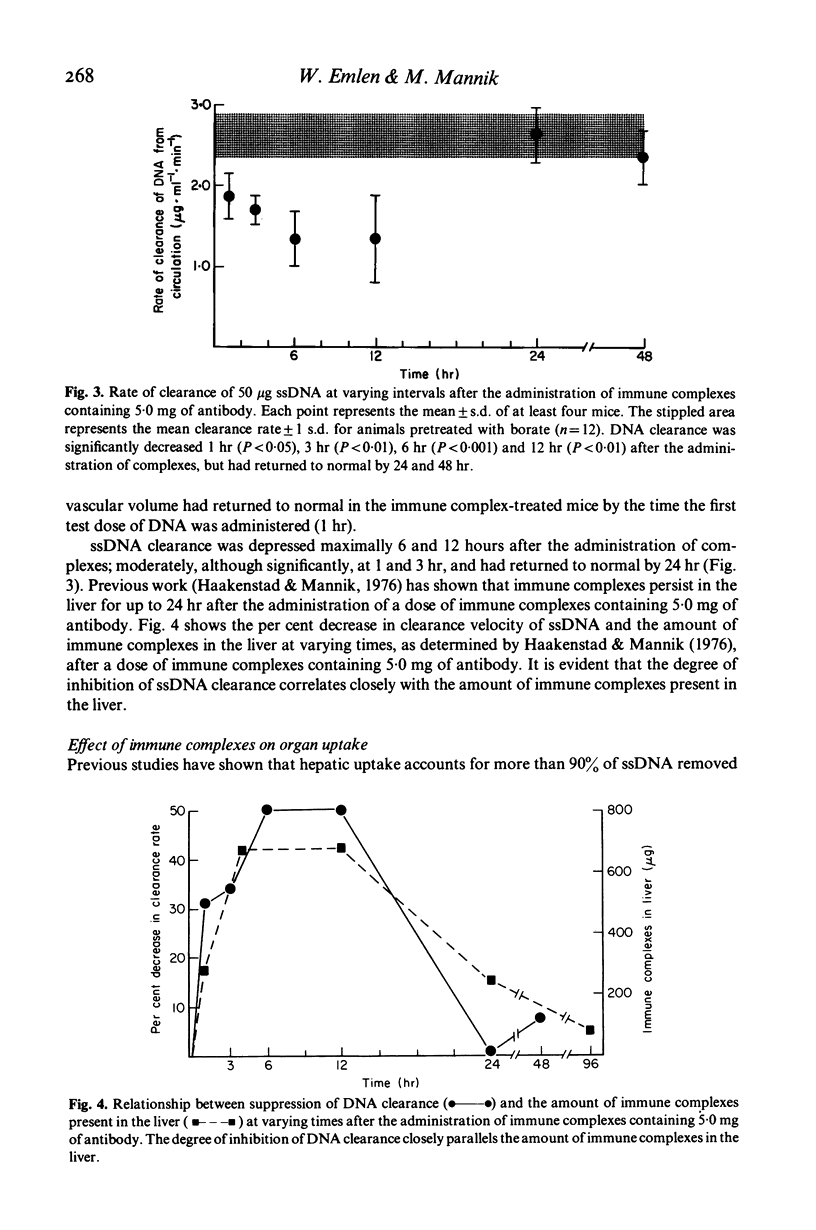
Recent studies have shown that DNA is cleared from the circulation extremely rapidly by the liver, and that normal individuals have low or immeasurable levels of circulating DNA. In some patients with SLE and in NZB/W mice, however, significant amounts of free DNA as well as DNA-anti-DNA immune complexes have been found in the circulation, suggesting a possible defect in DNA clearance in these conditions. To delineate factors which might contribute to the persistence of DNA in the circulation, we have assessed the effects of immune complexes on the clearance of single stranded DNA in normal C57Bl/6J mice. HSA-anti-HSA immune complexes at five-fold antigen excess were injected intravenously and after a variable, the clearance of single-stranded DNA was determined. Clearance of all doses of DNA was markedly suppressed 6 to 12 hr after the administration of immune complexes and returned to normal by 24 hr. Immune complexes decreased DNA clearance by blocking the hepatic uptake of DNA without altering the distribution of DNA to other organs. Histology and studies on the effect of immune complexes on the clearance of bromosulphophthalein (BSP) and sulphur colloid suggest that immune complexes affect DNA clearance by altering hepatic blood flow. The results obtained in this study suggest that circulating immune complexes in patients with SLE or in other conditions may suppress normal DNA clearance, and thereby contribute to the persistence of DNA in the circulation.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson J. P., Schreiber A. D., Frank M. M. Effects of corticosteroids and splenectomy on the immune clearance and destruction of erythrocytes. J Clin Invest. 1973 Jun;52(6):1509–1517. doi: 10.1172/JCI107325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano P. O., Jerry L. M., Sladowski J. P., Osterland C. K. Circulating immune complexes in systemic lupus erythematosus. Clin Exp Immunol. 1977 Aug;29(2):197–204. [PMC free article] [PubMed] [Google Scholar]
- Chused T. M., Steinberg A. D., Talal N. The clearance and localization of nucleic acids by New Zealand and normal mice. Clin Exp Immunol. 1972 Dec;12(4):465–476. [PMC free article] [PubMed] [Google Scholar]
- Commerford S. L. Iodination of nucleic acids in vitro. Biochemistry. 1971 May 25;10(11):1993–2000. doi: 10.1021/bi00787a005. [DOI] [PubMed] [Google Scholar]
- Dorsch C. A., Chia D., Levy L., Barnett E. V. Persistence of DNA in the circulation of immunized rabbits. J Rheumatol. 1975 Jun;2(2):161–166. [PubMed] [Google Scholar]
- Emlen W., Mannik M. Kinetics and mechanisms for removal of circulating single-stranded DNA in mice. J Exp Med. 1978 Mar 1;147(3):684–699. doi: 10.1084/jem.147.3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M. M., Hamburger M. I., Lawley T. J., Kimberly R. P., Plotz P. H. Defective reticuloendothelial system Fc-receptor function in systemic lupus erythematosus. N Engl J Med. 1979 Mar 8;300(10):518–523. doi: 10.1056/NEJM197903083001002. [DOI] [PubMed] [Google Scholar]
- Haakenstad A. O., Case J. B., Mannik M. Effect of cortisone on the disappearance kinetics and tissue localization of soluble immune complexes. J Immunol. 1975 Apr;114(4):1153–1160. [PubMed] [Google Scholar]
- Haakenstad A. O., Mannik M. Saturation of the reticuloendothelial system with soluble immune complexes. J Immunol. 1974 May;112(5):1939–1948. [PubMed] [Google Scholar]
- Haakenstad A. O., Mannik M. The disappearance kinetics of soluble immune complexes prepared with reduced and alkylated antibodies and with intact antibodies in mice. Lab Invest. 1976 Sep;35(3):283–292. [PubMed] [Google Scholar]
- Koffler D., Agnello V., Winchester R., Kunkel H. G. The occurrence of single-stranded DNA in the serum of patients with systemic lupus erythematosus and other diseases. J Clin Invest. 1973 Jan;52(1):198–204. doi: 10.1172/JCI107165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffler D., Carr R., Agnello V., Thoburn R., Kunkel H. G. Antibodies to polynucleotides in human sera: antigenic specificity and relation to disease. J Exp Med. 1971 Jul 1;134(1):294–312. doi: 10.1084/jem.134.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffler D., Schur P. H., Kunkel H. G. Immunological studies concerning the nephritis of systemic lupus erythematosus. J Exp Med. 1967 Oct 1;126(4):607–624. doi: 10.1084/jem.126.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan C., Kaplan M. H. Immunopathologic studies of systemic lupus erythematosus. II. Antinuclear reaction of gamma-globulin eluted from homogenates and isolated glomeruli of kidneys from patients with lupus nephritis. J Clin Invest. 1967 Apr;46(4):569–579. doi: 10.1172/JCI105558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert P. H., Dixon F. J. Pathogenesis of the glomerulonephritis of NZB/W mice. J Exp Med. 1968 Mar 1;127(3):507–522. doi: 10.1084/jem.127.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson S. M., Nelp W. B. Radiopharmacology of a simplifield technetium-99m-colloid preparation for photoscanning. J Nucl Med. 1966 Nov;7(11):817–826. [PubMed] [Google Scholar]
- Natali P. G., Tan E. M. Experimental renal disease induced by DNA-anti-DNA immune complexes. J Clin Invest. 1972 Feb;51(2):345–355. doi: 10.1172/JCI106820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normann S. J. Kinetics of phagocytosis. II. Analysis of in vivo clearance with demonstration of competitive inhibition between similar and dissimilar foreign particles. Lab Invest. 1974 Aug;31(2):161–169. [PubMed] [Google Scholar]
- SELIGSON D., MARINO J., DODSON E. Determination of sulfobromophthalein in serum. Clin Chem. 1957 Oct;3(5):638–645. [PubMed] [Google Scholar]
- Schmidt M. E., Douglas S. D. Disappearance and recovery of human monocyte IgG receptor activity after phagocytosis. J Immunol. 1972 Oct;109(4):914–917. [PubMed] [Google Scholar]
- Shand D. G. Drug disposition in liver disease. N Engl J Med. 1977 Jun 30;296(26):1527–1528. doi: 10.1056/NEJM197706302962612. [DOI] [PubMed] [Google Scholar]
- Steiner J. W. Investigations of Allergic Liver Injury: I. Light, Fluorescent and Electron Microscopic Study of the Effects of Soluble Immune Aggregates. Am J Pathol. 1961 Apr;38(4):411–436. [PMC free article] [PubMed] [Google Scholar]
- Steinman C. R., Ackad A. Appearance of circulating DNA during hemodialysis. Am J Med. 1977 May;62(5):693–697. doi: 10.1016/0002-9343(77)90872-5. [DOI] [PubMed] [Google Scholar]
- Steinman C. R. Free DNA in serum and plasma from normal adults. J Clin Invest. 1975 Aug;56(2):512–515. doi: 10.1172/JCI108118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Wilson C. B., Dixon F. J. The Raji cell radioimmune assay for detecting immune complexes in human sera. J Clin Invest. 1976 Jan;57(1):169–182. doi: 10.1172/JCI108257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfield J. B., Koffler D., Kunkel H. G. Role of DNA-anti-DNA complexes in the immunopathogenesis of tissue injury in systemic lupus erythematosus. Scand J Rheumatol Suppl. 1975;11:59–64. doi: 10.3109/03009747509095630. [DOI] [PubMed] [Google Scholar]
- Wood A. J., Villeneuve J. P., Branch R. A., Rogers L. W., Shand D. G. Intact hepatocyte theory of impaired drug metabolism in experimental cirrhosis in the rat. Gastroenterology. 1979 Jun;76(6):1358–1362. [PubMed] [Google Scholar]
- van Bezooijen C. F., Grell T., Knook D. L. Bromsulfophthalein uptake by isolated liver parenchymal cells. Biochem Biophys Res Commun. 1976 Mar 22;69(2):354–361. doi: 10.1016/0006-291x(76)90529-5. [DOI] [PubMed] [Google Scholar]


