Abstract
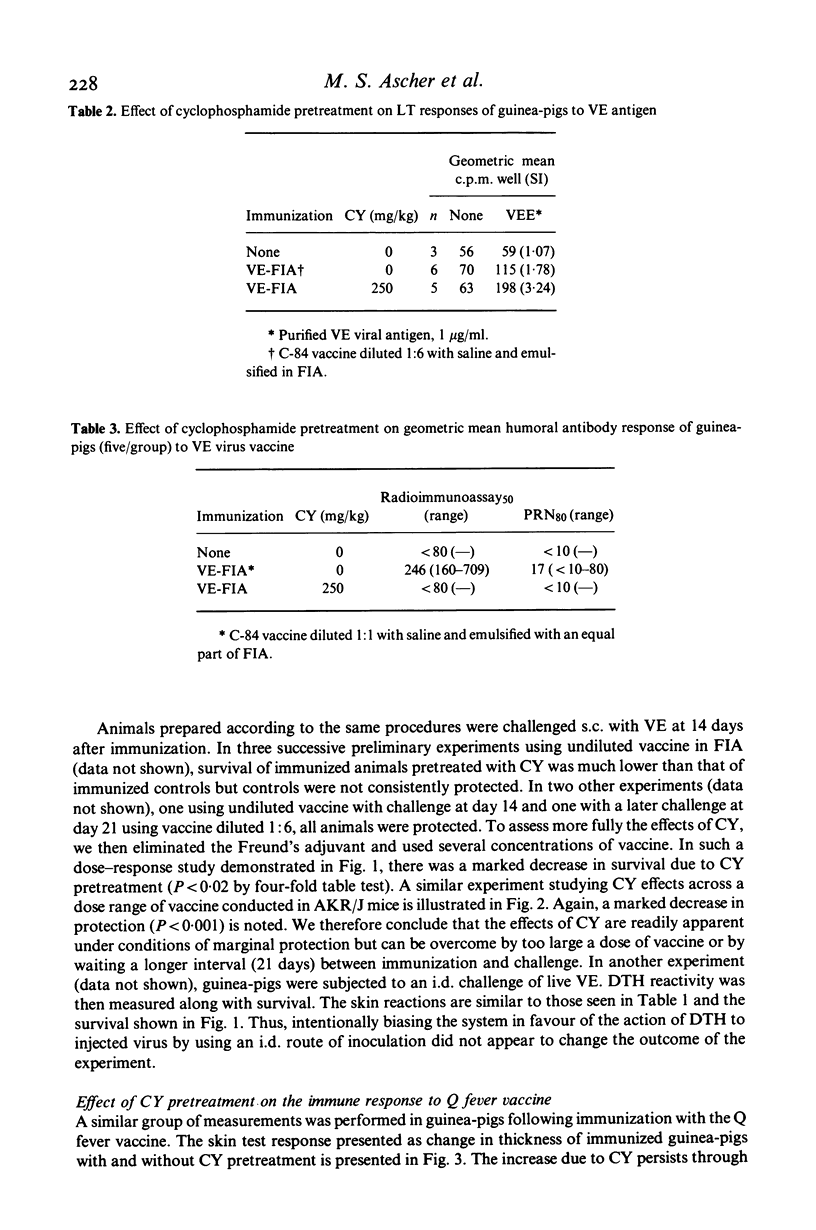
Administration of high-dose (250 mg/kg) cyclophosphamide (CY) to guinea-pigs and mice 3 days prior to immunization with inactivated vaccine derived from Venezuelan encephalitis virus (VE), Coxiella burnetii and Francisella tularensis resulted in accentuated and prolonged delayed-type hypersensitivity (DTH) and in vitro cellular immunity (CMI) to specific antigen. Humoral antibody were either absent or significantly lower in CY-pretreated animals compared to immunized non-pretreated controls. CY pretreatments precluded protection in the VE virus model, suggesting that resistance is related to antibody. In the Q fever model, the protective immunogenicity of vaccine was preserved or increased by CY pretreatment suggesting that cell-mediated immunity is the important factor. In the tularaemia bacterial system, there was a complex effect of CY pretreatment on the low-grade protection afforded by killed vaccine against virulent infection. These findings suggest that the inability of killed vaccines to induce high-grade resistance against tularaemia and Q fever may be due in part to a suppressive B cell response which is eliminated by CY. These studies have given useful information on the relative significance of components of the specific immune response and may lead to an increased understanding of the mechanisms of action of vaccines and adjuvants.
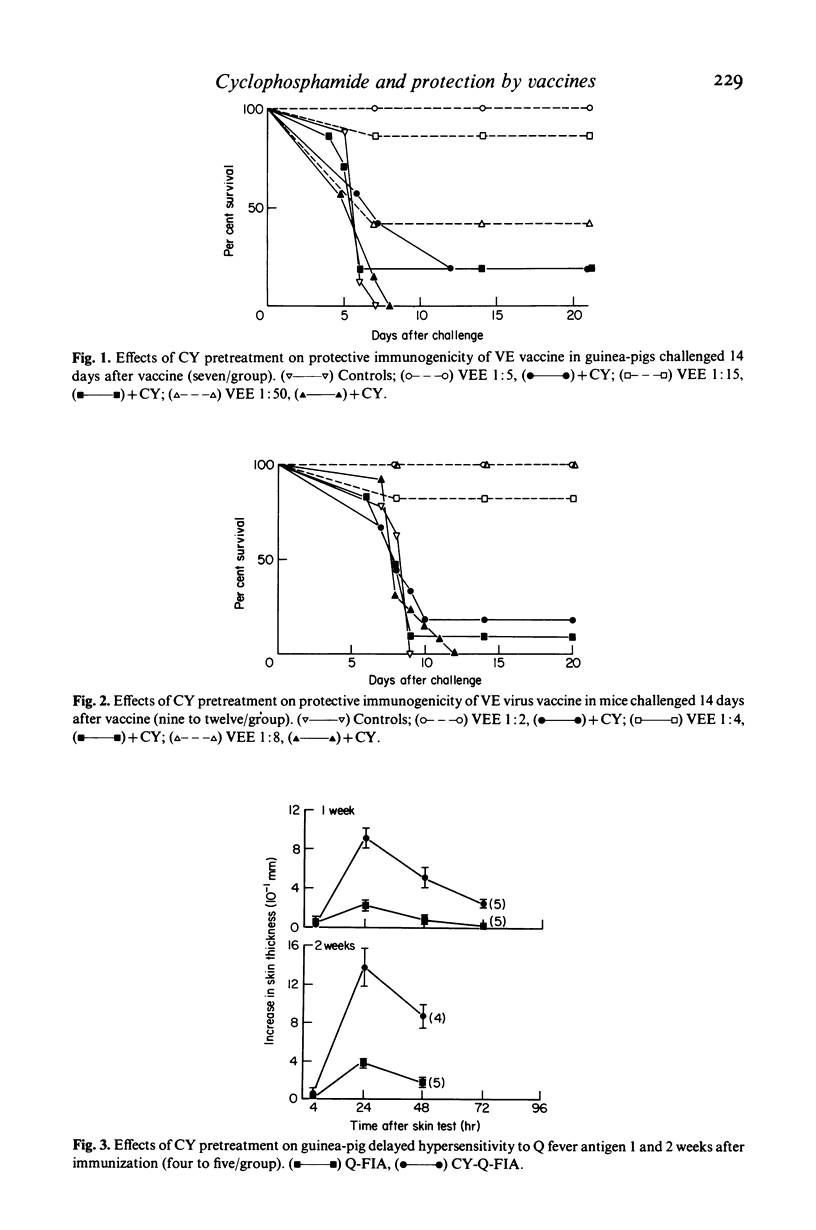
Full text
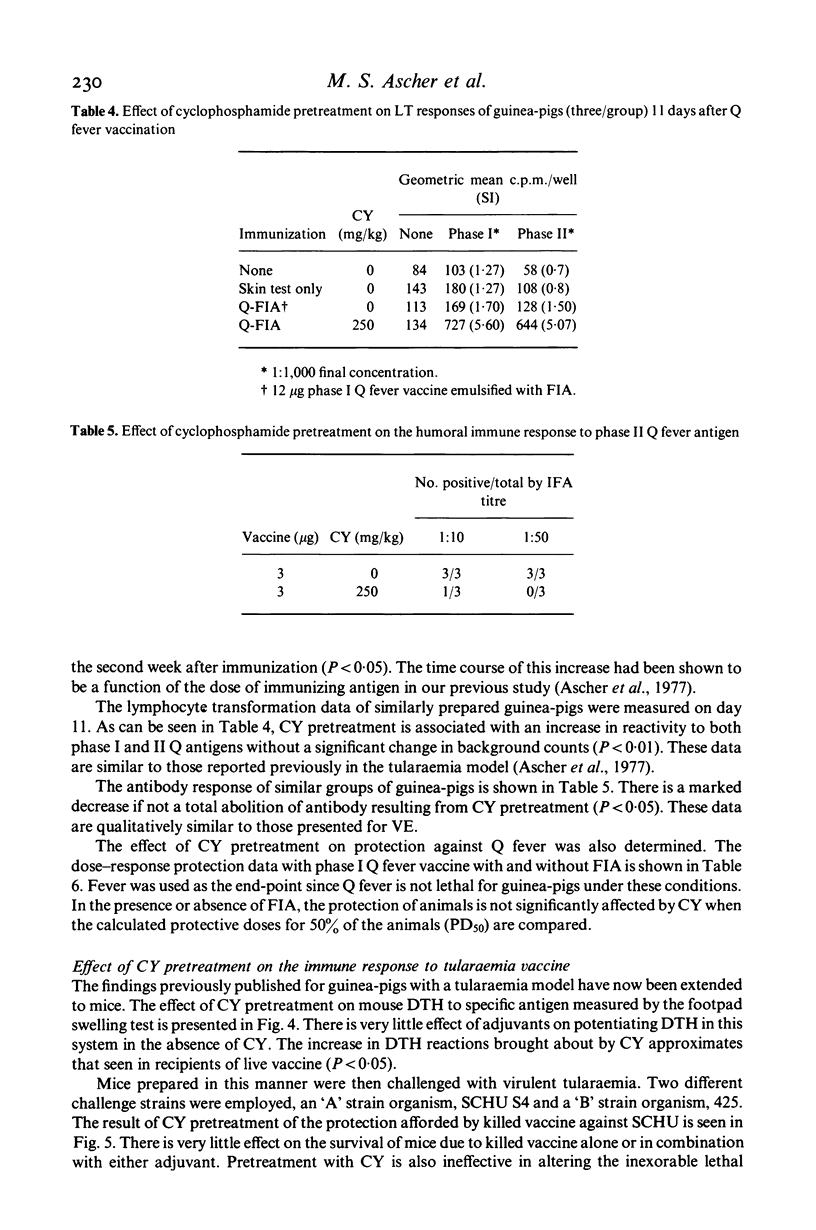
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABINANTI F. R., MARMION B. P. Protective or neutralizing antibody in Q fever. Am J Hyg. 1957 Sep;66(2):173–195. doi: 10.1093/oxfordjournals.aje.a119894. [DOI] [PubMed] [Google Scholar]
- ALLEN W. P. Immunity against tularemia: passive protection of mice by transfer of immune tissues. J Exp Med. 1962 Feb 1;115:411–420. doi: 10.1084/jem.115.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andron LA I. I., Eigelsbach H. T. Biochemical and immunological properties of ribonucleic acid-rich extracts from Francisella tularensis. Infect Immun. 1975 Jul;12(1):137–142. doi: 10.1128/iai.12.1.137-142.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araullo-Cruz T. P., Ho M., Armstrong J. A. Protective effect of early serum from mice after cytomegalovirus infection. Infect Immun. 1978 Sep;21(3):840–842. doi: 10.1128/iai.21.3.840-842.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher M. S., Parker D., Turk J. L. Modulation of delayed-type hypersensitivity and cellular immunity to microbial vaccines: effects of cyclophosphamide on the immune response to tularemia vaccine. Infect Immun. 1977 Nov;18(2):318–323. doi: 10.1128/iai.18.2.318-323.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELL J. F., LARSON C. L., WICHT W. C., RITTER S. A. Studies on the immunization of white mice against infections with Bacterium tularense. J Immunol. 1952 Nov;69(5):515–524. [PubMed] [Google Scholar]
- BERGE T. O., GLEISER C. A., GOCHENOUR W. S., Jr, MIESSE M. L., TIGERTT W. D. Studies on the virus of Venezuelan equine encephalomyelitis. II. Modification by specific immune serum of response of central nervous system of mice. J Immunol. 1961 Nov;87:509–517. [PubMed] [Google Scholar]
- BOZEMAN F. M., ELISBERG B. L. Serological diagnosis of scrub typhus by indirect immunofluorescence. Proc Soc Exp Biol Med. 1963 Mar;112:568–573. doi: 10.3181/00379727-112-28107. [DOI] [PubMed] [Google Scholar]
- Bascoul S., Cannat A., Huguet M. F., Serre A. Studies on the immune protection to murine experimental brucellosis conferred by Brucella fractions. I. Positive role of immune serum. Immunology. 1978 Aug;35(2):213–221. [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Maslan S. Effect of cyclophosphamide on experimental Nocardia asteroides infection in mice. Infect Immun. 1977 Jun;16(3):995–1004. doi: 10.1128/iai.16.3.995-1004.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belehu A., Poulter L. W., Turk J. L. Modification of cutaneous leishmaniasis in the guinea-pig by cyclophosphamide. Clin Exp Immunol. 1976 Apr;24(1):125–132. [PMC free article] [PubMed] [Google Scholar]
- Burke D. S. Immunization against tularemia: analysis of the effectiveness of live Francisella tularensis vaccine in prevention of laboratory-acquired tularemia. J Infect Dis. 1977 Jan;135(1):55–60. doi: 10.1093/infdis/135.1.55. [DOI] [PubMed] [Google Scholar]
- Catanzaro P. J., Shiral A., Agniel L. D., Jr, Osterman J. V. Host defenses in experimental scrub typhus: role of spleen and peritoneal exudate lymphocytes in cellular immunity. Infect Immun. 1977 Oct;18(1):118–123. doi: 10.1128/iai.18.1.118-123.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole F. E., Jr, May S. W., Eddy G. A. Inactivated Venezuelan equine encephalomyelitis vaccine prepared from attenuated (TC-83 strain) virus. Appl Microbiol. 1974 Jan;27(1):150–153. doi: 10.1128/am.27.1.150-153.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colizzi V., Garzelli C., Campa M., Falcone G. Depression of contact sensitivity by enhancement of suppressor cell activity in Pseudomonas aeruginosa-injected mice. Infect Immun. 1978 Aug;21(2):354–359. doi: 10.1128/iai.21.2.354-359.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozad G. C., Lindsey T. J. Effect of cyclophosphamide on Histoplasma capsulatum infections in mice. Infect Immun. 1974 Feb;9(2):261–265. doi: 10.1128/iai.9.2.261-265.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak H. F. Cutaneous basophil hypersensitivity. J Allergy Clin Immunol. 1976 Jul;58(1 Pt 2):229–240. doi: 10.1016/0091-6749(76)90159-7. [DOI] [PubMed] [Google Scholar]
- EIGELSBACH H. T., DOWNS C. M. Prophylactic effectiveness of live and killed tularemia vaccines. I. Production of vaccine and evaluation in the white mouse and guinea pig. J Immunol. 1961 Oct;87:415–425. [PubMed] [Google Scholar]
- Eigelsbach H. T., Hunter D. H., Janssen W. A., Dangerfield H. G., Rabinowitz S. G. Murine model for study of cell-mediated immunity: protection against death from fully virulent Francisella tularensis infection. Infect Immun. 1975 Nov;12(5):999–1005. doi: 10.1128/iai.12.5.999-1005.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finerty J. F., Krehl E. P. Cyclophosphamide pretreatment and protection against malaria. Infect Immun. 1976 Oct;14(4):1103–1105. doi: 10.1128/iai.14.4.1103-1105.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foshay L., Hesselbrock W. H., Wittenberg H. J., Rodenberg A. H. Vaccine Prophylaxis against Tularemia in Man. Am J Public Health Nations Health. 1942 Oct;32(10):1131–1145. doi: 10.2105/ajph.32.10.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON M. Studies on the relationship of delayed hypersensitivity to immunity in tularemia. J Immunol. 1963 Feb;90:209–216. [PubMed] [Google Scholar]
- Graybill J. R., Mitchell L. Cyclophosphamide effects on murine cryptococcosis. Infect Immun. 1978 Aug;21(2):674–677. doi: 10.1128/iai.21.2.674-677.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs D. J., Jerrells T. R. In vitro evaluation of immunity to Coxiella burnetii. J Immunol. 1976 Sep;117(3):996–1003. [PubMed] [Google Scholar]
- Jahrling P. B., Dendy E., Eddy G. A. Correlates to increased lethality of attenuated Venezuelan encephalitis virus vaccine for immunosuppressed hamsters. Infect Immun. 1974 May;9(5):924–930. doi: 10.1128/iai.9.5.924-930.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahrling P. B., Hesse R. A., Metzger J. F. Radioimmunoassay for quantitation of antibodies to alphaviruses with staphylococcal protein A. J Clin Microbiol. 1978 Jul;8(1):54–60. doi: 10.1128/jcm.8.1.54-60.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Mallavia L. P., Hinrichs D. J. Detection of long-term cellular immunity to Coxiella burneti as assayed by lymphocyte transformation. Infect Immun. 1975 Feb;11(2):280–286. doi: 10.1128/iai.11.2.280-286.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz S. I., Parker D., Turk J. L. B-cell suppression of delayed hypersensitivity reactions. Nature. 1974 Oct 11;251(5475):550–551. doi: 10.1038/251550a0. [DOI] [PubMed] [Google Scholar]
- Katz S. I., Parker D., Turk J. L. Mechanisms involved in the expression of Jones-Mote hypersensitivity. I. Passive cell transfer studies. Cell Immunol. 1975 Apr;16(2):396–403. doi: 10.1016/0008-8749(75)90128-8. [DOI] [PubMed] [Google Scholar]
- Kerckhaert J. A., Hofhuis F. M., Willers J. M. Effects of variation in time and dose of cyclophosphamide injection on delayed hypersensitivity and antibody formation. Cell Immunol. 1977 Mar 15;29(2):232–237. doi: 10.1016/0008-8749(77)90318-5. [DOI] [PubMed] [Google Scholar]
- Kerckhaert J. A., Hofhuis F. M., Willers J. M. Influence of cyclophosphamide on delayed hypersensitivity and acquired cellular resistance to Listeria monocytogenes in the mouse. Immunology. 1977 Jun;32(6):1027–1032. [PMC free article] [PubMed] [Google Scholar]
- Kishimoto R. A., Burger G. T. Appearance of cellular and humoral immunity in guinea pigs after infection with Coxiella burnetii administered in small-particle aerosols. Infect Immun. 1977 May;16(2):518–521. doi: 10.1128/iai.16.2.518-521.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto R. A., Johnson J. W., Kenyon R. H., Ascher M. S., Larson E. W., Pedersen C. E., Jr Cell-mediated immune responses of guinea pigs to an inactivated phase I Coxiella burnetii vaccine. Infect Immun. 1978 Jan;19(1):194–198. doi: 10.1128/iai.19.1.194-198.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto R. A., Rozmiarek H., Larson E. W. Experimental Q fever infection in congenitally athymic nude mice. Infect Immun. 1978 Oct;22(1):69–71. doi: 10.1128/iai.22.1.69-71.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto R. A., Veltri B. J., Shirey F. G., Canonico P. G., Walker J. S. Fat of Coxiella burnetti in macrophages from immune guinea pigs. Infect Immun. 1977 Feb;15(2):601–607. doi: 10.1128/iai.15.2.601-607.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto R. A., Walker J. S. Interaction between Coxiella burnetii and guinea pig peritoneal macrophages. Infect Immun. 1976 Aug;14(2):416–421. doi: 10.1128/iai.14.2.416-421.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostiala A. A., McGregor D. D., Logie P. S. Tularaemia in the rat. I. The cellular basis on host resistance to infection. Immunology. 1975 May;28(5):855–869. [PMC free article] [PubMed] [Google Scholar]
- LARSON C. L., BELL J. F., OWEN C. R. The development of resistance in mice immunized with soluble antigen derived from Bacterium tularense. J Immunol. 1954 Oct;73(4):221–225. [PubMed] [Google Scholar]
- Lagrange P. H., Tsiang H., Hurtrel B., Ravisse P. Delayed-type hypersensitivity to rabies virus in mice: assay of active or passive sensitization by the footpad test. Infect Immun. 1978 Sep;21(3):931–939. doi: 10.1128/iai.21.3.931-939.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc P. A., Scherer W. F., Sussdorf D. H. Infections of congenitally athymic (nude) and normal mice with avirulent and virulent strains of Venezuelan encephalitis virus. Infect Immun. 1978 Sep;21(3):779–785. doi: 10.1128/iai.21.3.779-785.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marker S. C., Ascher M. S. Specific in vitro lymphocyte transformation with Venezuelan equine encephalitis virus. Cell Immunol. 1976 Apr;23(1):32–38. doi: 10.1016/0008-8749(76)90169-6. [DOI] [PubMed] [Google Scholar]
- Miller A., Morse H. C., 3rd, Winkelstein J., Nathanson N. The role of antibody in recovery from experimental rabies. I. Effect of depletion of B and T cells. J Immunol. 1978 Jul;121(1):321–326. [PubMed] [Google Scholar]
- Modabber F., Bear S. E., Cerny J. The effect of cyclophosphamide on the recovery from a local chlamydial infection. Guinea-pig inclusion conjunctivitis (GPIC). Immunology. 1976 Jun;30(6):929–933. [PMC free article] [PubMed] [Google Scholar]
- Oakes J. E., Rosemond-Hornbeak H. Antibody-mediated recovery from subcutaneous herpes simplex virus type 2 infection. Infect Immun. 1978 Aug;21(2):489–495. doi: 10.1128/iai.21.2.489-495.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osebold J. W., Pearson L. D., Medin N. I. Relationship of antimicrobial cellular immunity to delayed hypersensitivity in Listeriosis. Infect Immun. 1974 Feb;9(2):354–362. doi: 10.1128/iai.9.2.354-362.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson C. L., Johnson A. G., Feller I. Effect of cyclophosphamide on the immune response to Pseudomonas aeruginosa in mice. Infect Immun. 1976 Jul;14(1):168–177. doi: 10.1128/iai.14.1.168-177.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz S. G., Adler W. H. Host defenses during primary Venezuelan equine encephalomyelitis virus infection in mice. I. Passive transfer of protection with immune serum and immune cells. J Immunol. 1973 May;110(5):1345–1353. [PubMed] [Google Scholar]
- Rabinowitz S. G. Host immune responses after administration of inactivated Venezuelan equine encephalomyelitis virus vaccines. I. Description and characterization of adoptive transfer by immune spleen cells. J Infect Dis. 1976 Jul;134(1):30–38. doi: 10.1093/infdis/134.1.30. [DOI] [PubMed] [Google Scholar]
- Rabinowitz S. G. Host immune responses after administration of inactivated Venezuelan equine encephalomyelitis virus vaccines. II. Kinetics of neutralizing antibody responses in donors and adoptively immunized recipients. J Infect Dis. 1976 Jul;134(1):39–47. doi: 10.1093/infdis/134.1.39. [DOI] [PubMed] [Google Scholar]
- Shepard C. C., Redus M. A. Effect of immunosuppressive drugs on infection in mice by M. marinum (balnei), M. tuberculosis and M. leprae. Int J Lepr Other Mycobact Dis. 1967 Jul-Sep;35(3):348–354. [PubMed] [Google Scholar]
- Shirai A., Catanzaro P. J., Phillips S. M., Osterman J. V. Host defenses in experimental scrub typhus: role of cellular immunity in heterologous protection. Infect Immun. 1976 Jul;14(1):39–46. doi: 10.1128/iai.14.1.39-46.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy S. P., Mackaness G. B. The effect of cytotoxic agents on the primary immune response to Listeria monocytogenes. J Exp Med. 1969 Jul 1;130(1):1–16. doi: 10.1084/jem.130.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODWARD J. M., STANSBERRY M. H., PRESSWOOD W. G., FOX J. B. CELLULAR IMMUNITY OF RATS TO TULAREMIA. Can J Microbiol. 1964 Aug;10:573–577. doi: 10.1139/m64-072. [DOI] [PubMed] [Google Scholar]
- Weiner L. P., Cole G. A., Nathanson N. Virus-specific immunologic depression in mice following combined immunization and cyclophosphamide-induced immunosuppression. J Immunol. 1971 Feb;106(2):427–430. [PubMed] [Google Scholar]
- Wells R. A., Diggs C. L., Phillips S. M. Cyclophosphamide-induced specific suppression of the protective immune response to rodent malaria. J Immunol. 1977 Feb;118(2):472–477. [PubMed] [Google Scholar]
- Woodman D. R., McManus A. T., Eddy G. A. Extension of the mean time to death of mice with a lethal infection of Venezuelan equine encephalomyelitis virus by antithymocyte serum treatment. Infect Immun. 1975 Nov;12(5):1006–1011. doi: 10.1128/iai.12.5.1006-1011.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


