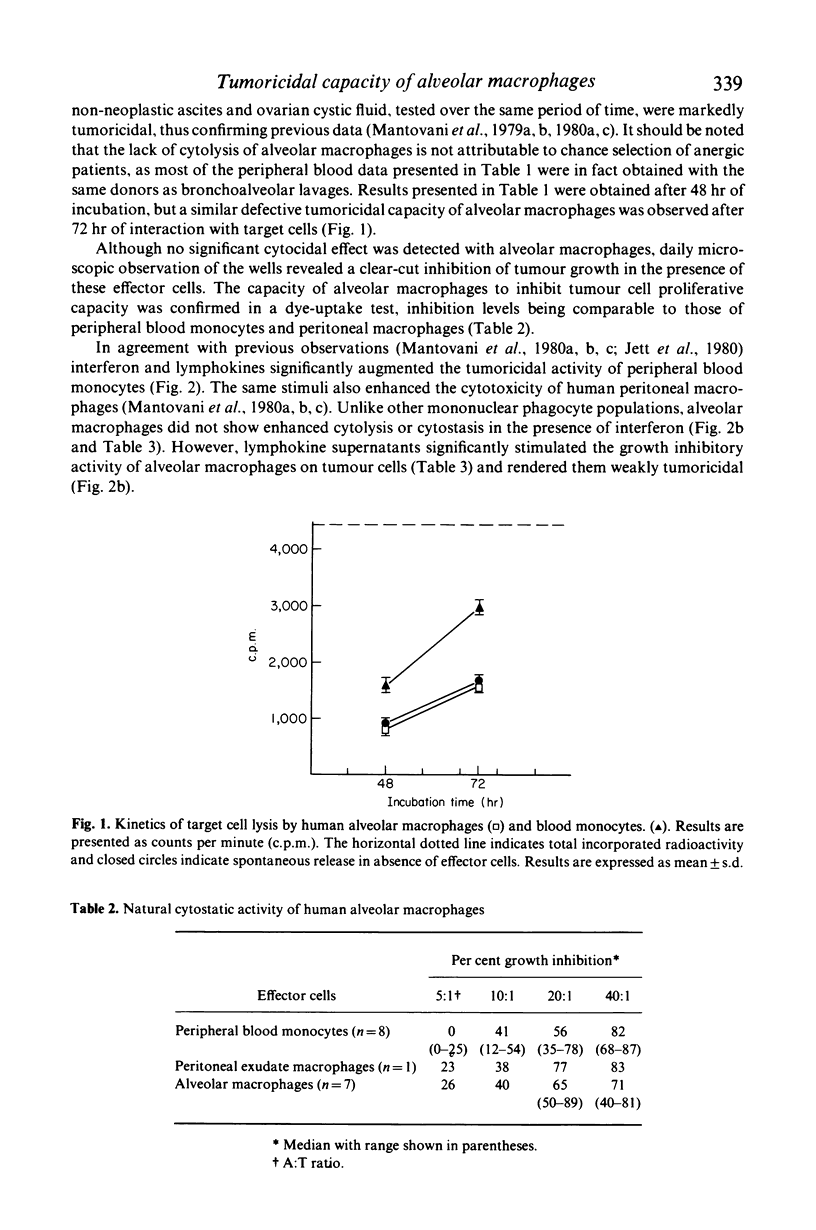
Abstract
Human cells of the monocyte-macrophage lineage were isolated by adherence from peripheral blood, peritoneal exudate, non-neoplastic ascites, benign ovarian cystic fluid and bronchoalveolar lavages. Cytolytic activity was measured as 3H-thymidine release from prelabelled mKSA TU5 tumour cells over 48-72 hr and cytostasis was evaluated in a 72-hr spectrophotometric assay. Mononuclear phagocytes from the various anatomical sites examined, except lung alveolar spaces, were significantly cytolytic and cytostatic on target cells. Unlike other cells of the monocyte-macrophage lineage, alveolar macrophages were not cytocidal, but significantly inhibited tumour cell proliferative capacity. Peripheral blood monocytes and peritoneal macrophages showed enhanced cytotoxicity in the presence of partially purified human fibroblast interferon or of lymphokine supernatants from mitogen-stimulated lymphocytes. In contrast, interferon did not affect the cytotoxic potential of alveolar macrophages, whereas lymphokines augmented their cytostatic activity and rendered them weakly cytolytic.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balkwill F. R., Hogg N. Characterization of human breast milk macrophages cytostatic for human cell lines. J Immunol. 1979 Oct;123(4):1451–1456. [PubMed] [Google Scholar]
- Cantrell E. T., Warr G. A., Busbee D. L., Martin R. R. Induction of aryl hydrocarbon hydroxylase in human pulmonary alveolar macrophages by cigarette smoking. J Clin Invest. 1973 Aug;52(8):1881–1884. doi: 10.1172/JCI107371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A. B., Cline M. J. The human alveolar macrophage: isolation, cultivation in vitro, and studies of morphologic and functional characteristics. J Clin Invest. 1971 Jul;50(7):1390–1398. doi: 10.1172/JCI106622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz D. A., Kight N., Temple A., Allison A. C. Spontaneous and induced cytotoxic properties of human adherent mononuclear cells: killing of non-sensitized and antibody-coated non-erythroid cells. Immunology. 1979 Feb;36(2):221–228. [PMC free article] [PubMed] [Google Scholar]
- Keller R. Macrophage-mediated natrual cytotoxicity against various target cells in vitro. I. Macrophages from diverse anatomical sites and different strains of rats and mice. Br J Cancer. 1978 May;37(5):732–741. doi: 10.1038/bjc.1978.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. Macrophage-mediated natural cytotoxicity against various target cells in vitro. II. Macrophages from rats of different ages. Br J Cancer. 1978 May;37(5):742–746. doi: 10.1038/bjc.1978.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Kurimura T., Dubbs D. R. Transplantable mouse tumor line induced by injection of SV40-transformed mouse kidney cells. Int J Cancer. 1969 Jul 15;4(4):384–392. doi: 10.1002/ijc.2910040403. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Bar Shavit Z., Peri G., Polentarutti N., Bordignon C., Sessa C., Mangioni C. Natural cytotoxicity on tumour cells of human macrophages obtained from diverse anatomical sites. Clin Exp Immunol. 1980 Mar;39(3):776–784. [PMC free article] [PubMed] [Google Scholar]
- Mantovani A., Jerrells T. R., Dean J. H., Herberman R. B. Cytolytic and cytostatic activity on tumor cells of circulating human monocytes. Int J Cancer. 1979 Jan 15;23(1):18–27. doi: 10.1002/ijc.2910230105. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Peri G., Polentarutti N., Bolis G., Mangioni C., Spreafico F. Effects on in vitro tumor growth of macrophages isolated from human ascitic ovarian tumors. Int J Cancer. 1979 Feb;23(2):157–164. doi: 10.1002/ijc.2910230204. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Tagliabue A., Dean J. H., Jerrells T. R., Herberman R. B. Cytolytic activity of circulating human monocytes on transformed and untransformed human fibroblasts. Int J Cancer. 1979 Jan 15;23(1):28–31. doi: 10.1002/ijc.2910230106. [DOI] [PubMed] [Google Scholar]
- Martin F., Martin M., Jeannin J. F., Lagneau A. Rat macrophage-mediated toxicity to cancer cells; effect of endotoxins and endotoxin inhibitors contained in culture media. Eur J Immunol. 1978 Aug;8(8):607–611. doi: 10.1002/eji.1830080813. [DOI] [PubMed] [Google Scholar]
- Meltzer M. S. Tumoricidal responses in vitro of peritoneal macrophages from conventionally housed and germ-free nude mice. Cell Immunol. 1976 Mar 1;22(1):176–181. doi: 10.1016/0008-8749(76)90018-6. [DOI] [PubMed] [Google Scholar]
- Miller G. A., Feldman J. D. Genetic role of rat macrophage cytotoxicity against tumor. Int J Cancer. 1976 Aug 15;18(2):168–175. doi: 10.1002/ijc.2910180206. [DOI] [PubMed] [Google Scholar]
- Schultz R. M., Pavlidis N. A., Stylos W. A., Chirigos M. A. Regulation of macrophage tumoricidal function: a role for prostaglandins of the E series. Science. 1978 Oct 20;202(4365):320–321. doi: 10.1126/science.694537. [DOI] [PubMed] [Google Scholar]
- Tagliabue A., Mantovani A., Kilgallen M., Herberman R. B., McCoy J. L. Natural cytotoxicity of mouse monocytes and macrophages. J Immunol. 1979 Jun;122(6):2363–2370. [PubMed] [Google Scholar]
- Weinberg J. B., Hibbs J. B., Jr Endocytosis of red blood cells or haemoglobin by activated macrophages inhibits their tumoricidal effect. Nature. 1977 Sep 15;269(5625):245–247. doi: 10.1038/269245a0. [DOI] [PubMed] [Google Scholar]


