Abstract
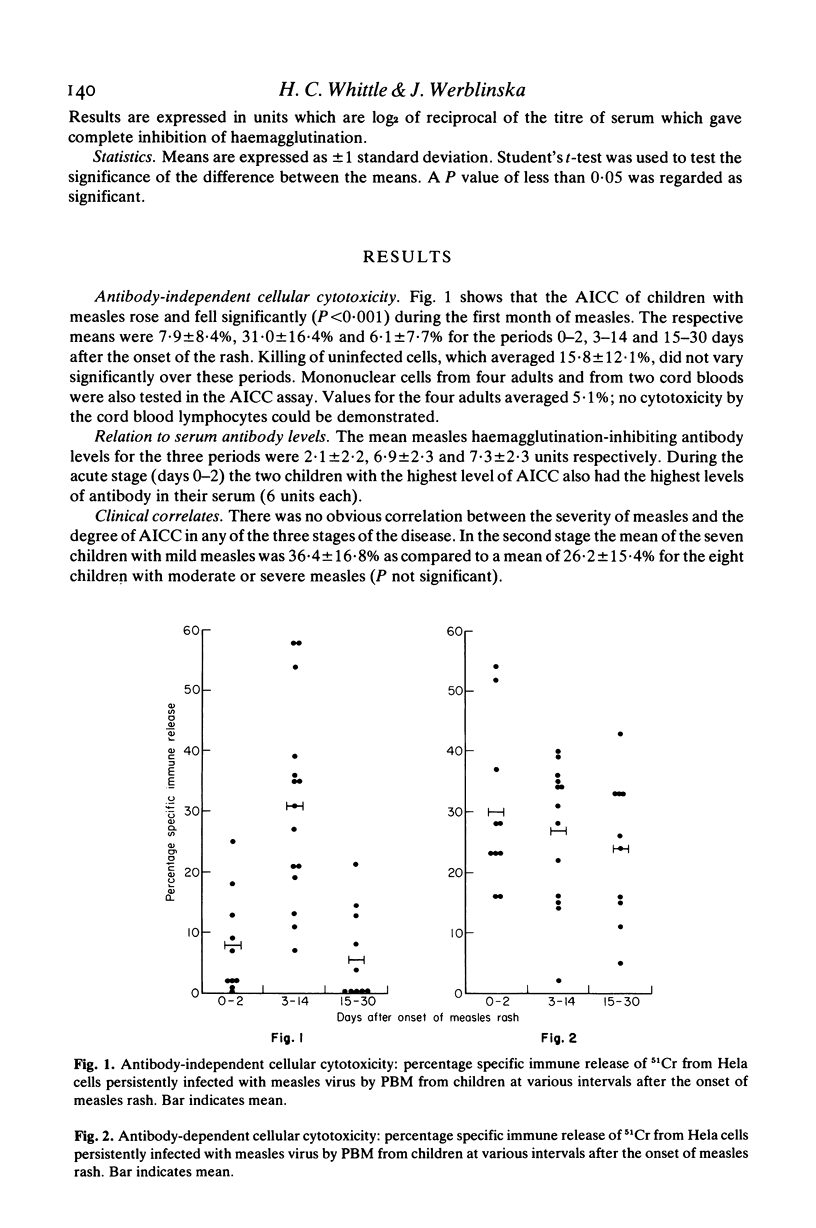
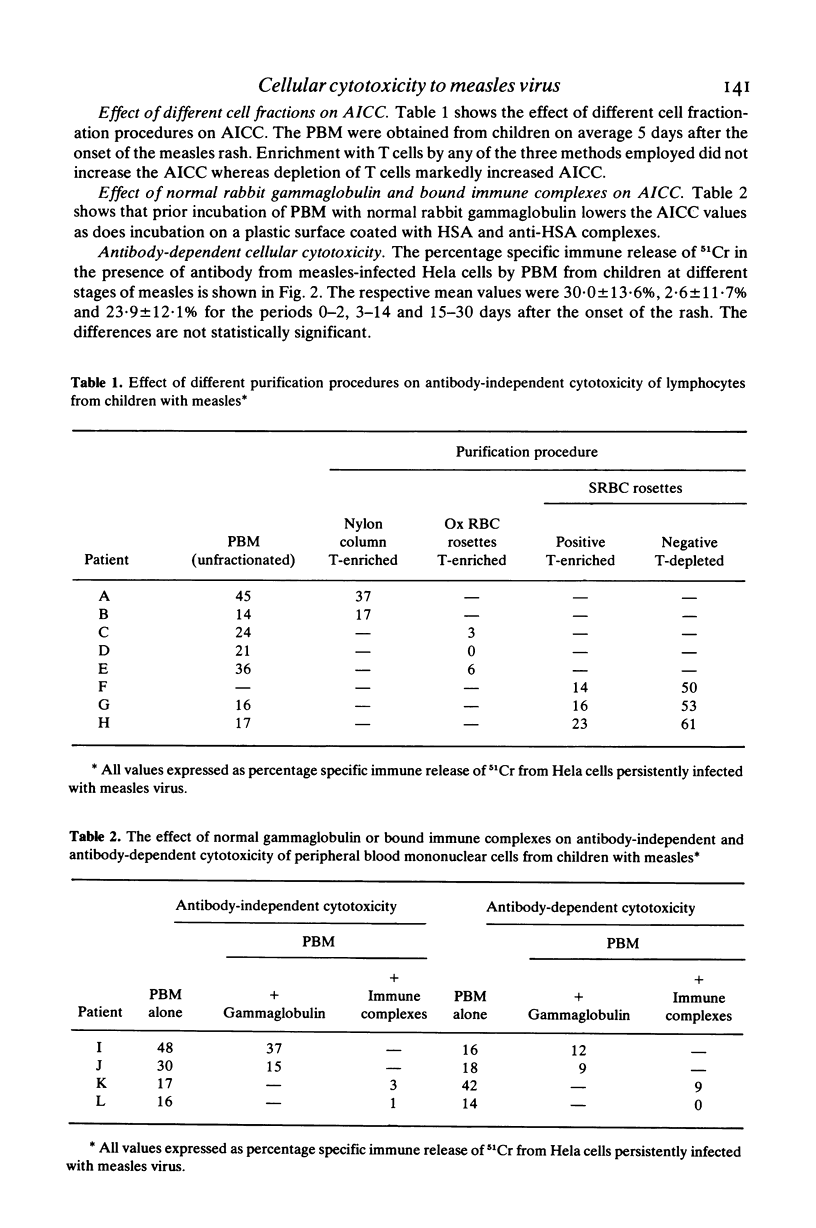
Little is known about cellular cytotoxicity to measles virus during natural measles infection which is still a major cause of death in many parts of the world. Therefore we measured the ability of peripheral blood mononuclear cells (PBM) from children with measles to kill Hela cells persistently infected with measles virus. In a 6-hr 51CR-release assay antibody-independent cellular cytotoxicity was shown to be low during the acute stage of measles. This rose to a maximum 1 week after the onset of the rash and fell rapidly on recovery 2 to 3 weeks later. The respective means values for the three periods (expressed as specific immune release of 51Cr) were 7·9±8·4%, 31·0±16·4% and 6·1±7·7%. Killing in this assay was not effected by T lymphocytes, for concentration of these cells by three different methods failed to increase cytotoxic power. In contrast peripheral blood mononuclear cells depleted of T lymphocytes showed greatly increased antibody-independent cellular cytotoxicity. Antibody-dependent cellular cytotoxicity was not found to vary significantly with the stage of measles. The mean values were 30·0±13·6%, 26·6±11·7% and 23·9±12·1% for the periods 0–2, 3–14 and 15–30 days after the onset of the rash. Both antibody-independent and antibody-dependent cellular cytotoxicity of PBM were lowered by layering these cells on immune complexes fixed to plastic or by incubating them with normal rabbit γ-globulin. Antibody-dependent cellular cytotoxicity was also lowered in the presence of 10% acute-phase autologous plasma. We concluded that antibody-independent cytoxocity was effected either by natural killer cells or by K cells using traces of antibody present in the assay. Antibody-dependent cellular cytotoxicity which is due to K cells may be modulated by circulating immune complexes during the course of disease.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ault K. A., Weiner H. L. Natural killing of measles-infected cells by human lymphocytes. J Immunol. 1979 Jun;122(6):2611–2616. [PubMed] [Google Scholar]
- Burnet F. M. Measles as an index of immunological function. Lancet. 1968 Sep 14;2(7568):610–613. doi: 10.1016/s0140-6736(68)90701-0. [DOI] [PubMed] [Google Scholar]
- Chiba S., Yamanaka T., Nakao T. Detection of cell-mediated immunity to measles virus with 51Cr release cytotoxic assay in patients with natural measles. Tohoku J Exp Med. 1974 Mar;112(3):285–288. doi: 10.1620/tjem.112.285. [DOI] [PubMed] [Google Scholar]
- ENDERS J. F., McCARTHY K., MITUS A., CHEATHAM W. J. Isolation of measles virus at autopsy in cases of giant-cell pneumonia without rash. N Engl J Med. 1959 Oct 29;261:875–881. doi: 10.1056/NEJM195910292611801. [DOI] [PubMed] [Google Scholar]
- Ewan P. W., Lachmann P. J. Demonstration of T-cell and K-cell cytotoxicity against measles-infected cells in normal subjects, multiple sclerosis and subacute sclerosing panencephalitis. Clin Exp Immunol. 1977 Oct;30(1):22–31. [PMC free article] [PubMed] [Google Scholar]
- GOOD R. A., ZAK S. J. Disturbances in gamma globulin synthesis as experiments of nature. Pediatrics. 1956 Jul;18(1):109–149. [PubMed] [Google Scholar]
- Herberman R. R., Ortaldo J. R., Bonnard G. D. Augmentation by interferon of human natural and antibody-dependent cell-mediated cytotoxicity. Nature. 1979 Jan 18;277(5693):221–223. doi: 10.1038/277221a0. [DOI] [PubMed] [Google Scholar]
- Hicks J. T., Sullivan J. L., Albrecht P. Immune responses during measles infection in immunosuppressed Rhesus monkeys. J Immunol. 1977 Oct;119(4):1452–1456. [PubMed] [Google Scholar]
- Joseph B. S., Oldstone M. B. Immunologic injury in measles virus infection. II. Suppression of immune injury through antigenic modulation. J Exp Med. 1975 Oct 1;142(4):864–876. doi: 10.1084/jem.142.4.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreth H. W., ter Meulen V., Eckert G. Demonstration of HLA restricted killer cells in patients with acute measles. Med Microbiol Immunol. 1979 Jan 24;165(4):203–214. doi: 10.1007/BF02152920. [DOI] [PubMed] [Google Scholar]
- Melewicz F. M., Shore S. L., Ades E. W., Phillips D. J. The mononuclear cell in human blood which mediates antibody-dependent cellular cytotoxicity to virus-infected target cells. II. Identification as a K cell. J Immunol. 1977 Feb;118(2):567–573. [PubMed] [Google Scholar]
- Morley D. Severe measles in the tropics. I. Br Med J. 1969 Feb 1;1(5639):297–contd. doi: 10.1136/bmj.1.5639.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahmias A. J., Griffith D., Salsbury C., Yoshida K. Thymic aplasia with lymphopenia, plasma cells, and normal immunoglobulins. Relation to measles virus infection. JAMA. 1967 Sep 4;201(10):729–734. [PubMed] [Google Scholar]
- Perrin L. H., Reynolds D., Zinkernagel R., Oldstone M. B. Generation of virus-specific cytolytic activity in human peripheral lymphocytes after vaccination with vaccinia virus and measles virus. Med Microbiol Immunol. 1978 Nov 17;166(1-4):71–79. doi: 10.1007/BF02121136. [DOI] [PubMed] [Google Scholar]
- Perrin L. H., Tishon A., Oldstone M. B. Immunologic injury in measles virus infection. III. Presence and characterization of human cytotoxic lymphocytes. J Immunol. 1977 Jan;118(1):282–290. [PubMed] [Google Scholar]
- Scheifele D. W., Forbes C. E. Prolonged giant cell excretion in severe African measles. Pediatrics. 1972 Dec;50(6):867–873. [PubMed] [Google Scholar]
- Valdirmarsson H., Agnarsdottir G., Lachmann P. J. Measles virus receptor on human T lymphocytes. Nature. 1975 Jun 12;255(5509):554–556. doi: 10.1038/255554a0. [DOI] [PubMed] [Google Scholar]
- Whittle H. C., Dossetor J., Oduloju A., Bryceson A. D., Greenwood B. M. Cell-mediated immunity during natural measles infection. J Clin Invest. 1978 Sep;62(3):678–684. doi: 10.1172/JCI109175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright L. L., Levy N. L. Generation on infected fibroblasts of human T and non-T lymphocytes with specific cytotoxicity, influenced by histocompatibility, against measles virus-infected cells. J Immunol. 1979 Jun;122(6):2379–2387. [PubMed] [Google Scholar]
- Zinkernagel R. M., Althage A. Antiviral protection by virus-immune cytotoxic T cells: infected target cells are lysed before infectious virus progeny is assembled. J Exp Med. 1977 Mar 1;145(3):644–651. doi: 10.1084/jem.145.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M. Major transplantation antigens in T cell-mediated immunity: a comparison of the transplantation reaction with antiviral immunity. Fed Proc. 1978 Aug;37(10):2379–2384. [PubMed] [Google Scholar]


