Abstract
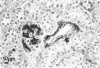
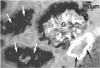
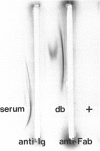
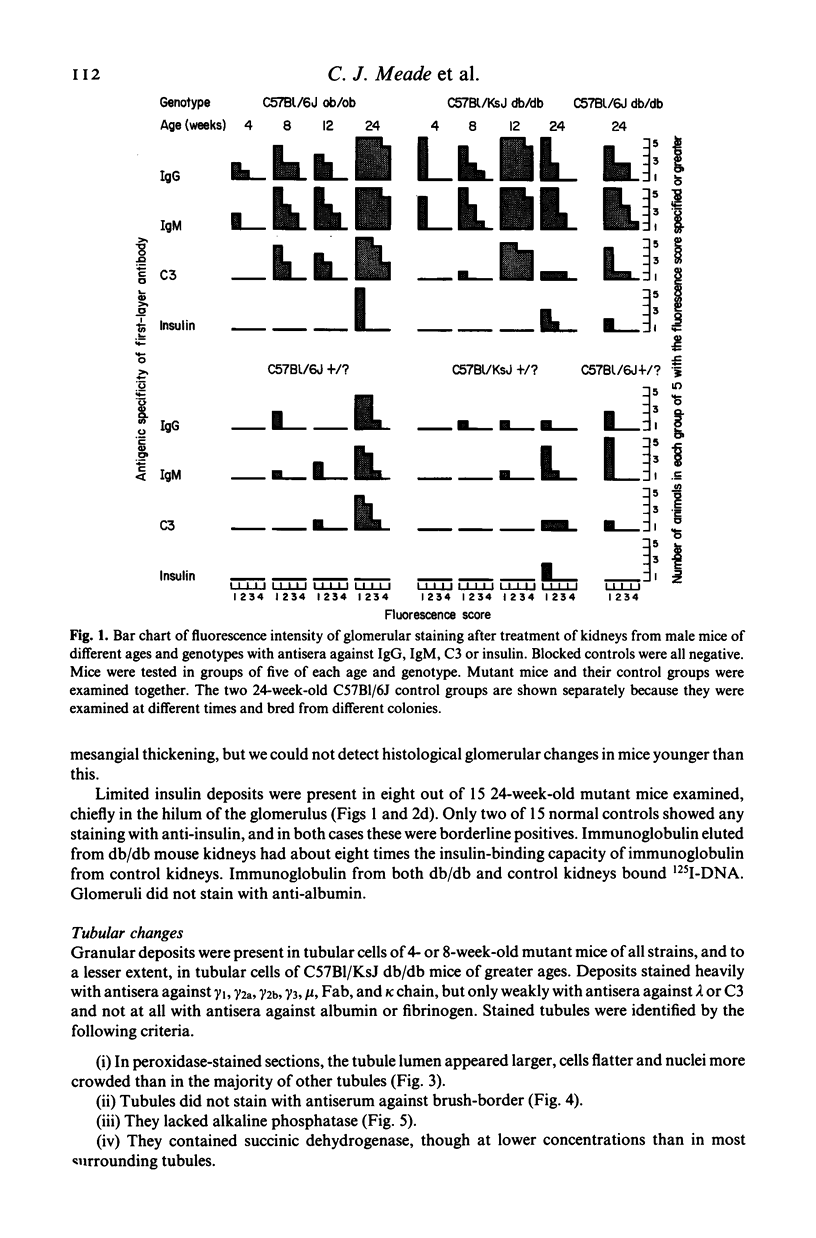
Kidney lesions were studied by light microscopy and immunofluorescence in diabetic (db/db) and obese (ob/ob) mutant mice. The db/db mutation was studied both on the C57Bl/KsJ genetic background (where it produces severe hyperglycaemia) and on the C57Bl/6J background (where hyperglycaemia is only mild). In all cases, more IgG, IgM and C3 were deposited in the renal glomeruli of mutant mice than in the glomeruli of normal (+/?) mice of equivalent age. First signs of immunoglobulin deposition occurred at a slightly younger age than first signs of C3 deposition or histological change (mainly mesangial thickening). Insulin deposits were occasionally seen in the glomeruli of older mutant mice and immunoglobulin eluted from diabetic mouse kidneys had anti-insulin activity. Increased anti-DNA activity was present in the serum of older mutants. In those mutants with severe hyperglycaemia, the macula densa and distal convoluted tubules also contained immunoglobulin deposits, probably derived from the glomerular mesangium. Urine from diabetic mice contained high molecular weight material reacting with antisera to Fab or kappa but not the Fc portion of immunoglobulin. We conclude that diabetic mice have immune complexes in the kidney containing antibodies against insulin and possibly other antigens. We find no evidence that hyperglycaemia itself is the direct cause of glomerular immune complex deposition, although there may be a link between hyperglycaemia and tubular dysfunction.
Full text
PDF


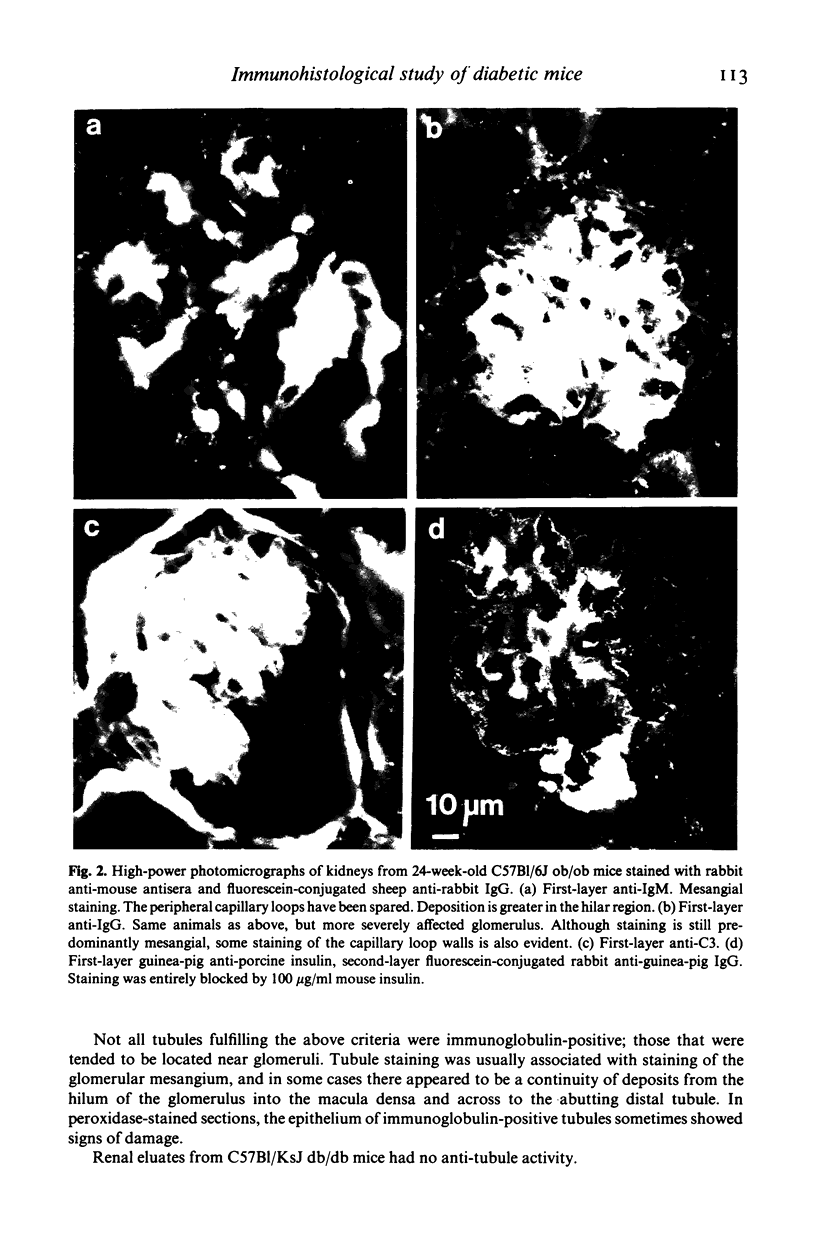
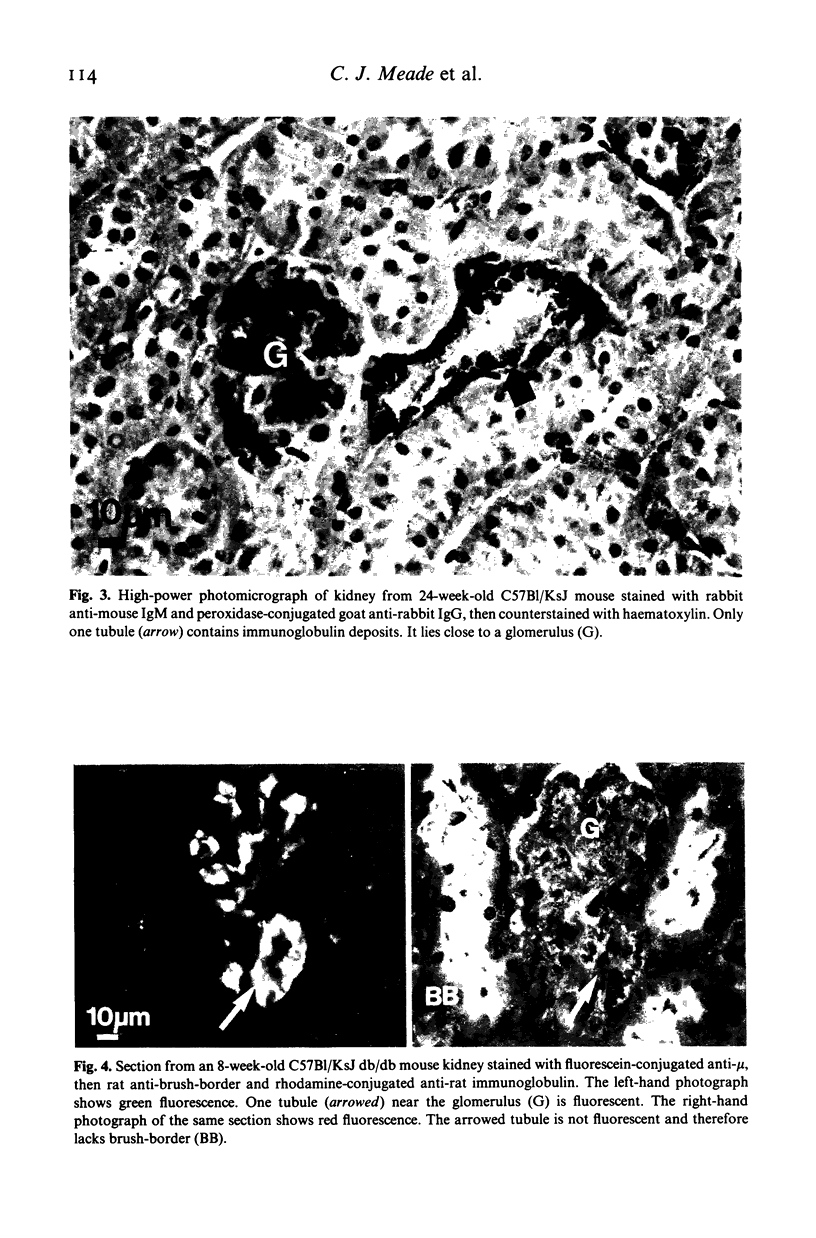

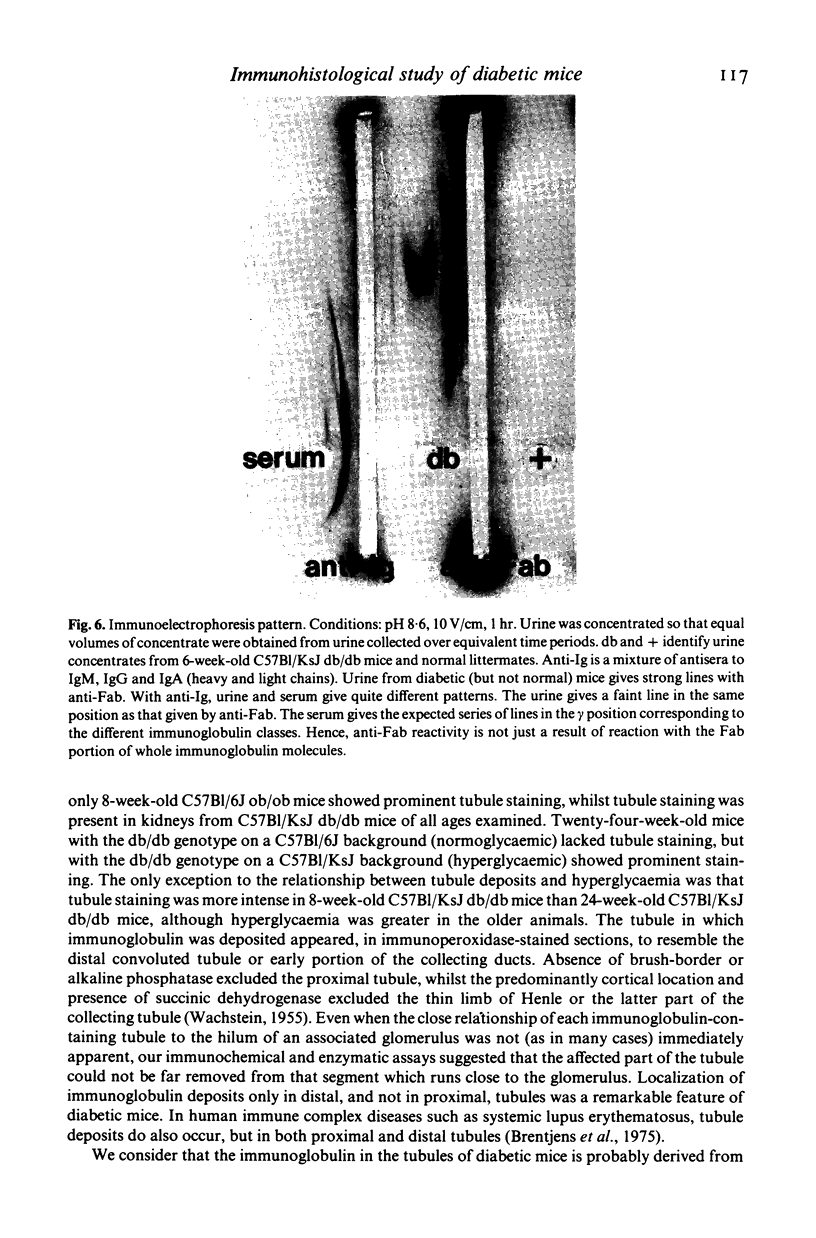



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNS A. W., OWENS C. T., HIRATA Y., BLUMENTHAL H. T. The pathogenesis of diabetic glomerulosclerosis. II. A demonstration of insulin-binding capacity of the various histopathological components of the disease by fluorescence microscopy. Diabetes. 1962 Jul-Aug;11:308–317. [PubMed] [Google Scholar]
- BLUMENTHAL H. T., BERNS A. W., OWENS C. T., HIRATA Y. The pathogenesis of diabetic glomerulosclerosis. I. The significance of various histopathological components of the disease. Diabetes. 1962 Jul-Aug;11:296–307. [PubMed] [Google Scholar]
- Bartolotti S. R. Quantitative elution studies in experimental immune complex and nephrotoxic nephritis. Clin Exp Immunol. 1977 Aug;29(2):334–341. [PMC free article] [PubMed] [Google Scholar]
- Bergstrand A., Nathorst-Windahl G., Hellman B. The electron microscopic appearance of the glomerular lesions in obese-hyperglycaemic mice. Acta Pathol Microbiol Scand. 1968;74(2):161–168. doi: 10.1111/j.1699-0463.1968.tb03467.x. [DOI] [PubMed] [Google Scholar]
- Brentjens J. R., Sepulveda M., Baliah T., Bentzel C., Erlanger B. F., Elwood C., Montes M., Hsu K. C., Andres G. A. Interstitial immune complex nephritis in patients with systemic lupus erythematosus. Kidney Int. 1975 May;7(5):342–350. doi: 10.1038/ki.1975.47. [DOI] [PubMed] [Google Scholar]
- Burkholder P. M. Immunohistopathologic study of localized plasma proteins and fixation of guinea pig complement in renal lesions of diabetic glomerulosclerosis. Diabetes. 1965 Dec;14(12):755–770. doi: 10.2337/diab.14.12.755. [DOI] [PubMed] [Google Scholar]
- Burns J. Background staining and sensitivity of the unlabelled antibody-enzyme (PAP) method. Comparison with the peroxidase labelled antibody sandwich method using formalin fixed paraffin embedded material. Histochemistry. 1975 Jun 5;43(3):291–294. doi: 10.1007/BF00499711. [DOI] [PubMed] [Google Scholar]
- Coleman D. L. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978 Mar;14(3):141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- Comerford F. R., Cohen A. S., Desai R. G. The evolution of the glomerular lesion in NZB mice. A light and electron microscopic study. Lab Invest. 1968 Dec;19(6):643–651. [PubMed] [Google Scholar]
- Elema J. D., Hoyer J. R., Vernier R. L. The glomerular mesangium: uptake and transport of intravenously injected colloidal carbon in rats. Kidney Int. 1976 May;9(5):395–406. doi: 10.1038/ki.1976.49. [DOI] [PubMed] [Google Scholar]
- FARRANT P. C., SHEDDEN W. I. OBSERVATIONS ON THE UPTAKE OF INSULIN CONJUGATED WITH FLUORESCEIN ISOTHIOCYANATE BY DIABETIC KIDNEY TISSUE. Diabetes. 1965 May;14:274–278. doi: 10.2337/diab.14.5.274. [DOI] [PubMed] [Google Scholar]
- Fernandes G., Handwerger B. S., Yunis E. J., Brown D. M. Immune response in the mutant diabetic C57BL/Ks-dt+ mouse. Discrepancies between in vitro and in vivo immunological assays. J Clin Invest. 1978 Feb;61(2):243–250. doi: 10.1172/JCI108933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehman D. H., Wilson C. B., Dixon F. J. Extraglomerular immunoglobulin deposits in human nephritis. Am J Med. 1975 Jun;58(6):765–796. doi: 10.1016/0002-9343(75)90632-4. [DOI] [PubMed] [Google Scholar]
- Leiper J. M., Thomson D., MacDonald M. K. Uptake and transport of Imposil by the glomerular mesangium in the mouse. Lab Invest. 1977 Nov;37(5):526–533. [PubMed] [Google Scholar]
- Like A. A., Lavine R. L., Poffenbarger P. L., Chick W. L. Studies in the diabetic mutant mouse. VI. Evolution of glomerular lesions and associated proteinuria. Am J Pathol. 1972 Feb;66(2):193–224. [PMC free article] [PubMed] [Google Scholar]
- Mahmoud A. A., Rodman H. M., Mandel M. A., Warren K. S. Induced and spontaneous diabetes mellitus and suppression of cell-mediated immunologic responses. Granuloma formation, delayed dermal reactivity and allograft rejection. J Clin Invest. 1976 Feb;57(2):362–367. doi: 10.1172/JCI108287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Mauer S. M., Steffes M. W., Michael A. F., Brown D. M. Studies of diabetic nephropathy in animals and man. Diabetes. 1976;25(2 Suppl):850–857. [PubMed] [Google Scholar]
- Meade C. J., Sheena J., Mertin J. Effects of the obese (ob/ob) genotype on spleen cell immune function. Int Arch Allergy Appl Immunol. 1979;58(2):121–127. doi: 10.1159/000232183. [DOI] [PubMed] [Google Scholar]
- NACHLAS M. M., TSOU K. C., DE SOUZA E., CHENG C. S., SELIGMAN A. M. Cytochemical demonstration of succinic dehydrogenase by the use of a new p-nitrophenyl substituted ditetrazole. J Histochem Cytochem. 1957 Jul;5(4):420–436. doi: 10.1177/5.4.420. [DOI] [PubMed] [Google Scholar]
- NATHORST-WINDAHL G., HELLMAN B. LIPOHYALIN GLOMERULAR LESIONS IN AGEING OBESE-HYPERGLYCEMIC MICE. Med Exp Int J Exp Med. 1964;10:67–71. doi: 10.1159/000135396. [DOI] [PubMed] [Google Scholar]
- Pincus T. Immunochemical conditions affecting the measurement of DNA antibodies using ammonium sulfate precipitation. Arthritis Rheum. 1971 Sep-Oct;14(5):623–630. doi: 10.1002/art.1780140509. [DOI] [PubMed] [Google Scholar]
- RUEMKE P., THUNG P. J. IMMUNOLOGICAL STUDIES ON THE SEX-DEPENDENT PREALBUMIN IN MOUSE URINE AND ON ITS OCCURRENCE IN THE SERUM. Acta Endocrinol (Copenh) 1964 Sep;47:156–164. doi: 10.1530/acta.0.0470156. [DOI] [PubMed] [Google Scholar]
- Reading M. A digestion technique for the reduction of background staining in the immunoperoxidase method. J Clin Pathol. 1977 Jan;30(1):88–90. doi: 10.1136/jcp.30.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheena J., Meade C. J. Mice bearing the ob/ob mutation have impaired immunity. Int Arch Allergy Appl Immunol. 1978;57(3):263–268. doi: 10.1159/000232111. [DOI] [PubMed] [Google Scholar]
- Striker G. E., Mannik M., Tung M. Y. Role of marrow-derived monocytes and mesangial cells in removal of immune complexes from renal glomeruli. J Exp Med. 1979 Jan 1;149(1):127–136. doi: 10.1084/jem.149.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinder P. Determination of blood glucose using 4-amino phenazone as oxygen acceptor. J Clin Pathol. 1969 Mar;22(2):246–246. doi: 10.1136/jcp.22.2.246-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WACHSTEIN M. Histochemical staining reactions of the normally functioning and abnormal kidney. J Histochem Cytochem. 1955 Jul;3(4):246–270. doi: 10.1177/3.4.246. [DOI] [PubMed] [Google Scholar]
- Westberg N. G., Michael A. F. Immunohistopathology of diabetic glomerulosclerosis. Diabetes. 1972 Mar;21(3):163–174. doi: 10.2337/diab.21.3.163. [DOI] [PubMed] [Google Scholar]