Abstract
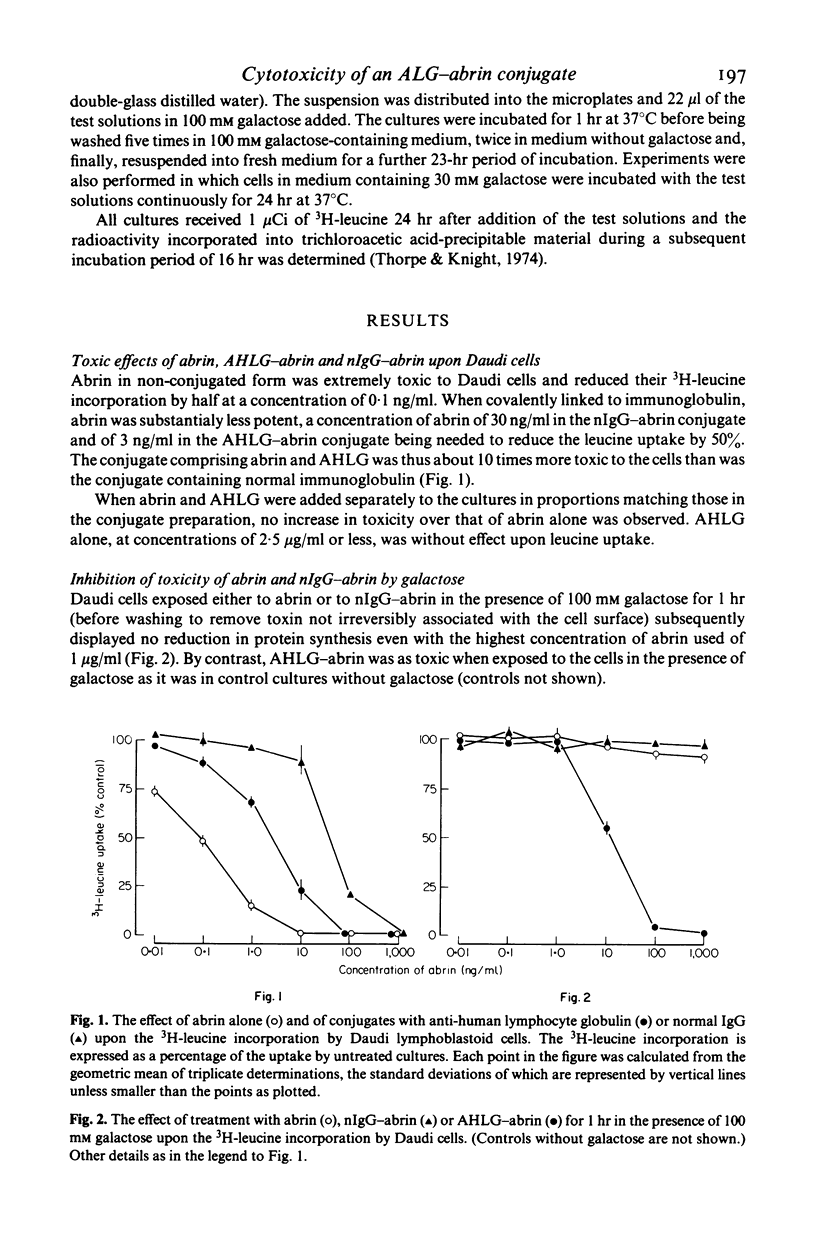
A covalent conjugate of abrin and anti-human lymphocyte globulin (AHLG) was prepared in an endeavour to create a cytotoxic agent with specificity for human lymphoid cells. The AHLG--abrin conjugate was found to be around 10-fold better able to inhibit 3H-leucine uptake by the human lymphoblastoid cell line, Daudi, in tissue culture than was the control conjugate comprising abrin and normal IgG (nIgG). Both materials were less potent than native abrin. Galactose, which is known competitively to antagonize the binding of abrin to cells, strongly inhibited the toxicities of abrin and the nIgG--abrin conjugate whereas that of ALG--abrin was unimpaired. Thus, at least for Daudi cells in tissue culture, abrin can be made selectively toxic, by linkage to AHLG, towards cells bearing antigens to which the antibody moiety of the conjugate can attach.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolten F. L., Capparell N. J., Cooperband S. R. Antitumor effects of antibody-diphtheria toxin conjugates: use of hapten-coated tumor cells as an antigenic target. J Natl Cancer Inst. 1972 Oct;49(4):1057–1062. [PubMed] [Google Scholar]
- Moolten F. L., Capparell N. J., Zajdel S. H., Cooperband S. R. Antitumor effects of antibody-diphtheria toxin conjugates. II. Immunotherapy with conjugates directed against tumor antigens induced by simian virus 40. J Natl Cancer Inst. 1975 Aug;55(2):473–477. [PubMed] [Google Scholar]
- Moolten F., Zajdel S., Cooperband S. Immunotherapy of experimental animal tumors with antitumor antibodies conjugated to diphtheria toxin or ricin. Ann N Y Acad Sci. 1976;277(00):690–699. doi: 10.1111/j.1749-6632.1976.tb41740.x. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Pihl A. Isolation and properties of abrin: a toxic protein inhibiting protein synthesis. Evidence for different biological functions of its two constituent-peptide chains. Eur J Biochem. 1973 May;35(1):179–185. doi: 10.1111/j.1432-1033.1973.tb02823.x. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Refsnes K., Pihl A. Mechanism of action of the toxic lectins abrin and ricin. Nature. 1974 Jun 14;249(458):627–631. doi: 10.1038/249627a0. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Sandvig K., Refsnes K., Pihl A. Rates of different steps involved in the inhibition of protein synthesis by the toxic lectins abrin and ricin. J Biol Chem. 1976 Jul 10;251(13):3985–3992. [PubMed] [Google Scholar]
- ROSS W. C. In vitro reactions of biological alkylating agents. Ann N Y Acad Sci. 1958 Apr 24;68(3):669–681. doi: 10.1111/j.1749-6632.1958.tb42633.x. [DOI] [PubMed] [Google Scholar]
- Ross W. C., Thorpe P. E., Cumber A. J., Edwards D. C., Hinson C. A., Davies A. J. Increased toxicity of diphtheria toxin for human lymphoblastoid cells following covalent linkage to anti-(human lymphocyte) globulin or its F(ab')2 fragment. Eur J Biochem. 1980 Mar;104(2):381–390. doi: 10.1111/j.1432-1033.1980.tb04438.x. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S., Pihl A. Chemical modifications of the toxic lectins abrin and ricin. Eur J Biochem. 1978 Mar 15;84(2):323–331. doi: 10.1111/j.1432-1033.1978.tb12171.x. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S., Pihl A. Kinetics of binding of the toxic lectins abrin and ricin to surface receptors of human cells. J Biol Chem. 1976 Jul 10;251(13):3977–3984. [PubMed] [Google Scholar]
- Thorpe P. E., Knight S. C. Microplate culture of mouse lymph node cells. I. Quantitation of responses to allogeneic lymphocytes endotoxin and phytomitogens. J Immunol Methods. 1974 Oct;5(4):387–404. doi: 10.1016/0022-1759(74)90022-2. [DOI] [PubMed] [Google Scholar]
- Thorpe P. E., Ross W. C., Cumber A. J., Hinson C. A., Edwards D. C., Davies A. J. Toxicity of diphtheria toxin for lymphoblastoid cells is increased by conjugation to antilymphocytic globulin. Nature. 1978 Feb 23;271(5647):752–755. doi: 10.1038/271752a0. [DOI] [PubMed] [Google Scholar]
- Woiwod A. J., Courtenay J. S., Edwards D. C., Epps H. B., Knight R. R., Mosedale B., Phillips A. W., Rahr L., Thomas D., Woodrooffe J. G. The preparation and properties of horse antihuman lymphocyte serum and globulin. Transplantation. 1970 Aug;10(2):173–186. doi: 10.1097/00007890-197008000-00004. [DOI] [PubMed] [Google Scholar]
- Woodruff M. F., Reid B. L., James K. Quantitative studies with antilymphocytic antibody. Nature. 1967 Nov 25;216(5117):758–762. doi: 10.1038/216758a0. [DOI] [PubMed] [Google Scholar]
- Youle R. J., Murray G. J., Neville D. M., Jr Ricin linked to monophosphopentamannose binds to fibroblast lysosomal hydrolase receptors, resulting in a cell-type-specific toxin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5559–5562. doi: 10.1073/pnas.76.11.5559. [DOI] [PMC free article] [PubMed] [Google Scholar]


