Abstract
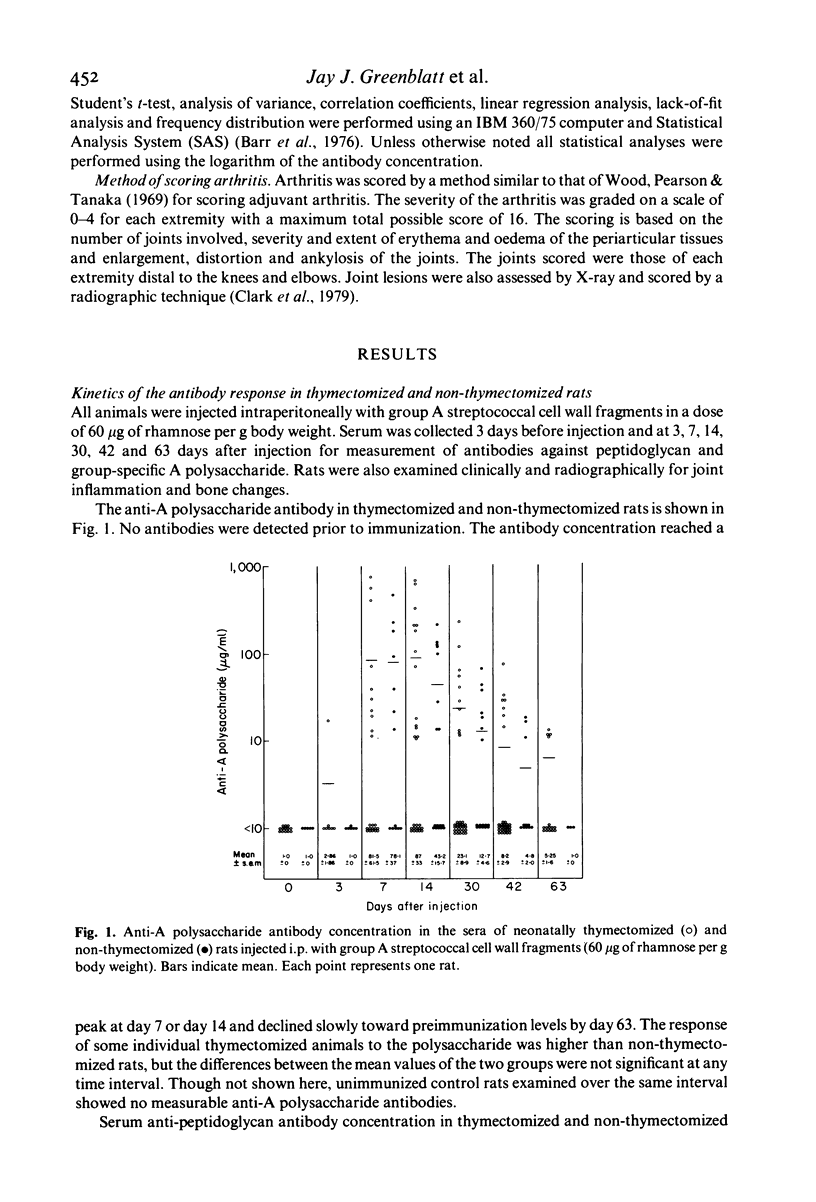
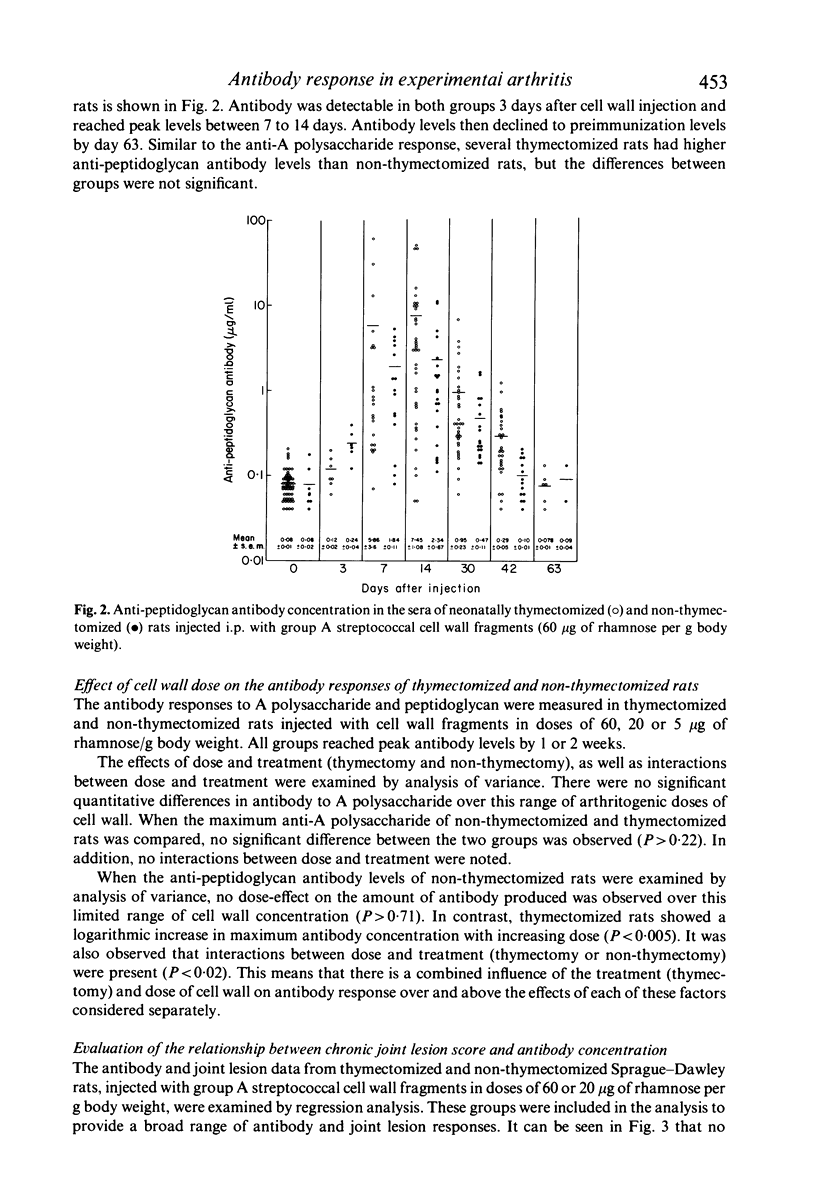
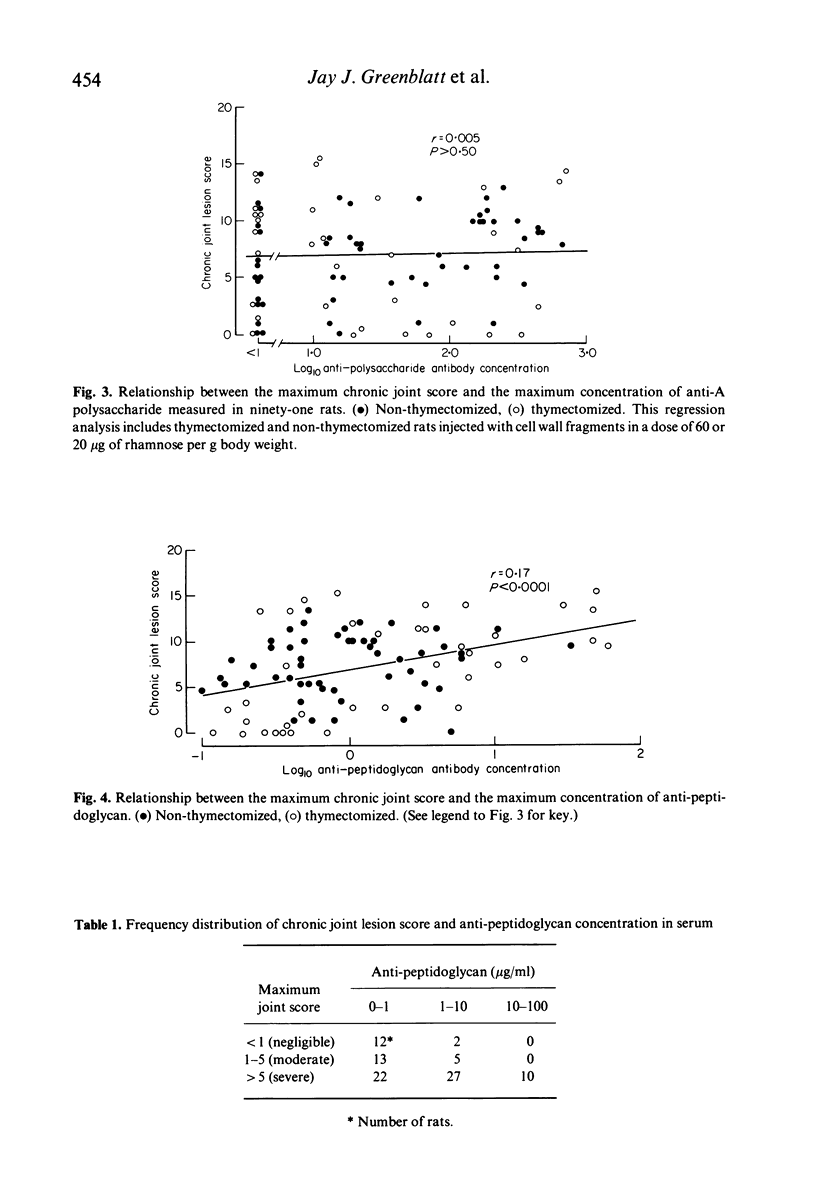
The antibody response to group A streptococcal cell wall components was measured in rats during the development of chronic, remittent experimental arthritis. The arthritis was induced by a single intraperitoneal injection of an aqueous suspension of group A streptococcal cell wall fragments and antibodies were measured by a radioactive antigen-binding assay. Antibodies in serum against both peptidoglycan and A polysaccharide reached maximum levels at 1 or 2 weeks and declined to preimmunization levels by day 63. The kinetics and magnitude of the antibody responses were similar in neonatally thymectomized and non-thymectomized rats. A relationship between chronic joint lesions and anti-peptidoglycan concentration in serum was indicated, since all rats which produced high levels of antibody developed severe chronic arthritis. However, 46% of the rats which produced very low levels of antibody also developed moderate to severe arthritis. There was no correlation between anti-A polysaccharide antibodies and joint disease, although the concentration of this antibody was 10- to 100-fold greater than the anti-peptidoglycan. We conclude that antibody can be a component in the pathogenesis of this experimental model of arthritis, but its role requires further elucidation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Braun D. G., Kindred B., Jacobson E. B. Streptococcal group A carbohydrate antibodies in mice: evidence for strain differences in magnitude and restriction of the response, and for thymus dependence. Eur J Immunol. 1972 Apr;2(2):138–143. doi: 10.1002/eji.1830020209. [DOI] [PubMed] [Google Scholar]
- Clark R. L., Cuttino J. T., Jr, Anderle S. K., Cromartie W. J., Schwab J. H. Radiologic analysis of arthritis in rats after systemic injection of streptococcal cell walls. Arthritis Rheum. 1979 Jan;22(1):25–35. doi: 10.1002/art.1780220105. [DOI] [PubMed] [Google Scholar]
- Cromartie W. J., Craddock J. G., Schwab J. H., Anderle S. K., Yang C. H. Arthritis in rats after systemic injection of streptococcal cells or cell walls. J Exp Med. 1977 Dec 1;146(6):1585–1602. doi: 10.1084/jem.146.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damais C., Bona C., Chedid L., Fleck J., Nauciel C., Martin J. P. Mitogenic effect of bacterial peptidoglycans possessing adjuvant activity. J Immunol. 1975 Jul;115(1):268–271. [PubMed] [Google Scholar]
- Davies P., Page R. C., Allison A. C. Changes in cellular enzyme levels and extracellular release of lysosomal acid hydrolases in macrophages exposed to group A streptococcal cell wall substance. J Exp Med. 1974 May 1;139(5):1262–1282. doi: 10.1084/jem.139.5.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichmann K., Braun D. G., Krause R. M. Influence of genetic factors on the magnitude and the heterogeneity of the immune response in the rabbit. J Exp Med. 1971 Jul 1;134(1):48–65. doi: 10.1084/jem.134.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHER E. R., FISHER B. ROLE OF THYMUS IN SKIN AND TUMOR TRANSPLANTATION IN THE RAT. Lab Invest. 1965 May;14:546–555. [PubMed] [Google Scholar]
- Gotschlich E. C. A simplification of the radioactive antigen-binding test by a double label technique. J Immunol. 1971 Sep;107(3):910–911. [PubMed] [Google Scholar]
- Gotschlich E. C., Rey M., Triau R., Sparks K. J. Quantitative determination of the human immune response to immunization with meningococcal vaccines. J Clin Invest. 1972 Jan;51(1):89–96. doi: 10.1172/JCI106801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt J. J., Eichmann K., Braun D., Krause R. M. Factors that enhance the potency of streptococcal group-specific antisera. J Infect Dis. 1971 Oct;124(4):387–393. doi: 10.1093/infdis/124.4.387. [DOI] [PubMed] [Google Scholar]
- Greenblatt J., Boackle R. J., Schwab J. H. Activation of the alternate complement pathway by peptidoglycan from streptococcal cell wall. Infect Immun. 1978 Jan;19(1):296–303. doi: 10.1128/iai.19.1.296-303.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymer B., Bernstein D., Schleifer K. H., Krause R. M. A radioactive hapten-binding assay for measuring antibodies to the pentapeptide determinant of peptidoglycan. J Immunol. 1975 Apr;114(4):1191–1196. [PubMed] [Google Scholar]
- Heymer B., Schleifer K. H., Read S., Zabriskie J. B., Krause R. M. Detection of antibodies to bacterial cell wall peptidoglycan in human sera. J Immunol. 1976 Jul;117(1):23–26. [PubMed] [Google Scholar]
- Hunter N., Anderle S. K., Brown R. R., Dalldorf F. G., Clark R. L., Cromartie W. J., Schwab J. H. Cell-mediated immune response during experimental arthritis induced in rats with streptococcal cell walls. Clin Exp Immunol. 1980 Dec;42(3):441–449. [PMC free article] [PubMed] [Google Scholar]
- Jones J. M., Amsbaugh D. F., Prescott B. Kinetics of the antibody response to type III pneumococcal polysaccharide (SSS-III). I. Use of 125I-labeled SSS-III to study serum antibody levels, as well as the distribution and excretion of antigen after immunization. J Immunol. 1976 Jan;116(1):41–51. [PubMed] [Google Scholar]
- KRAUSE R. M., MCCARTY M. Studies on the chemical structure of the streptococcal cell wall. I. The identification of a mucopeptide in the cell walls of groups A and A-variant streptococci. J Exp Med. 1961 Jul 1;114:127–140. doi: 10.1084/jem.114.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbel R. S., Eidinger D. Enhanced immune responsiveness to a thymus-independent antigen early after adult thymectomy: evidence for short-lived inhibitory thymus-derived cells. Eur J Immunol. 1972 Apr;2(2):114–118. doi: 10.1002/eji.1830020204. [DOI] [PubMed] [Google Scholar]
- Leslie G. A., Carwile H. F. Immune response of rats to group A streptococcal vaccine. Infect Immun. 1973 May;7(5):781–785. doi: 10.1128/iai.7.5.781-785.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolicka M., Massell B. F., Wilgram G. F. Antipeptidoglycan in rheumatic fever: agreement with carditis. Proc Soc Exp Biol Med. 1973 Dec;144(3):892–895. doi: 10.3181/00379727-144-37705. [DOI] [PubMed] [Google Scholar]
- Schorlemmer H. U., Bitter-Suermann D., Allison A. C. Complement activation by the alternative pathway and macrophage enzyme secretion in the pathogenesis of chronic inflammation. Immunology. 1977 Jun;32(6):929–940. [PMC free article] [PubMed] [Google Scholar]
- Smialowicz R. J., Schwab J. H. Cytotoxicity of rat macrophages activated by persistent or biodegradable bacterial cell walls. Infect Immun. 1977 Sep;17(3):599–606. doi: 10.1128/iai.17.3.599-606.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood F. D., Pearson C. M., Tanaka A. Capacity of mycobacterial wax D and its subfractions to induce adjuvant arthritis in rats. Int Arch Allergy Appl Immunol. 1969;35(5):456–467. doi: 10.1159/000230198. [DOI] [PubMed] [Google Scholar]


