Abstract
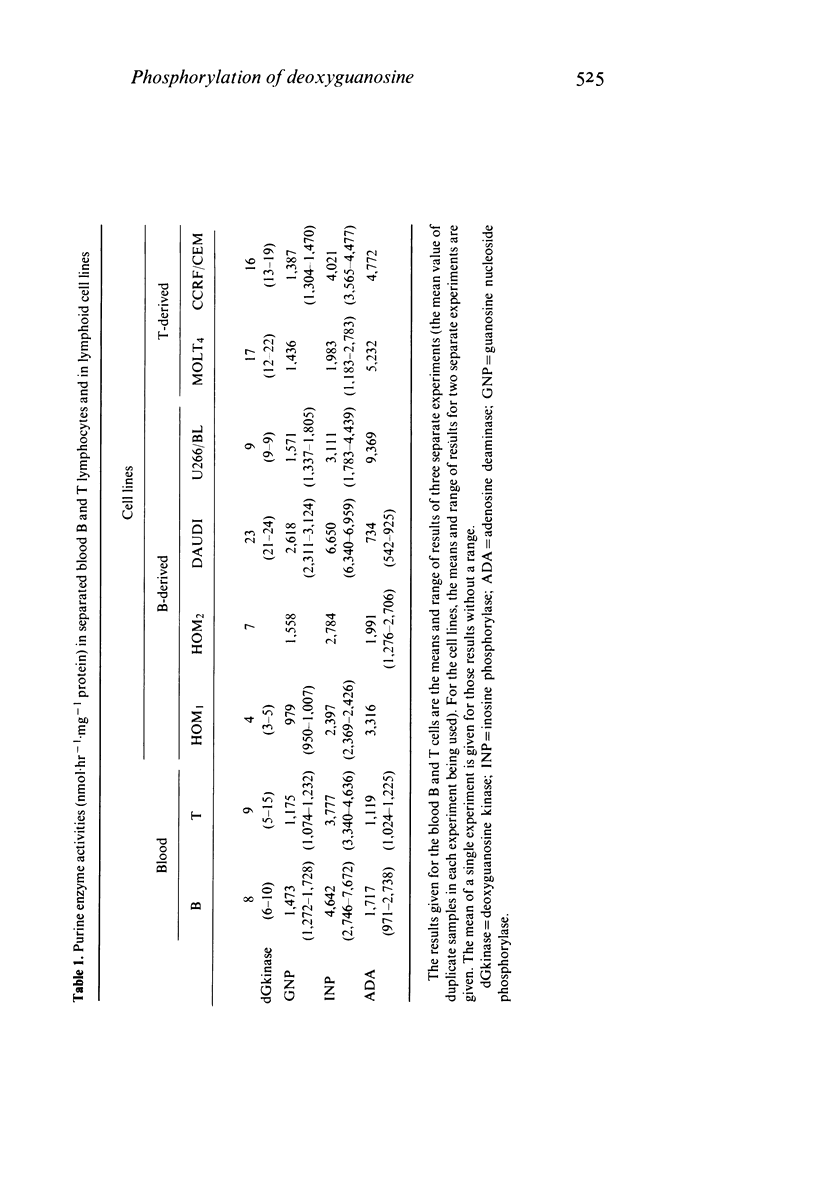
T and B lymphocytes from blood and tonsils, as well as T and B lymphoid cell lines, phosphorylate deoxyguanosine which is probably the major toxic metabolite in purine nucleoside phosphorylase (PNP) deficiency. T and B cells also have similar activities of purine nucleoside phosphorylase. These findings indicate that the selective T cell defect in patients with PNP deficiency is unlikely to be due to a selective 'trapping' of deoxyguanosine by T lymphocytes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carson D. A., Kaye J., Seegmiller J. E. Lymphospecific toxicity in adenosine deaminase deficiency and purine nucleoside phosphorylase deficiency: possible role of nucleoside kinase(s). Proc Natl Acad Sci U S A. 1977 Dec;74(12):5677–5681. doi: 10.1073/pnas.74.12.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
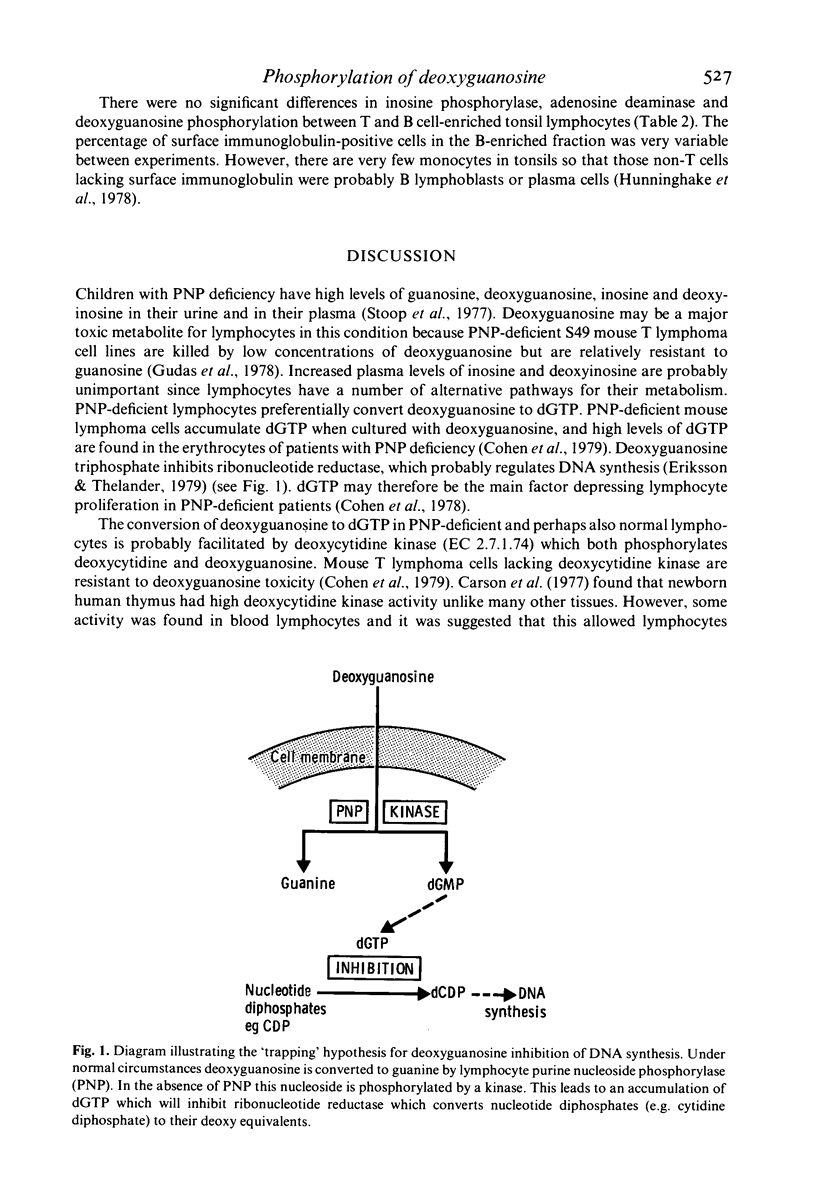
- Cohen A., Gudas L. J., Ammann A. J., Staal G. E., Martin D. W., Jr Deoxyguanosine triphosphate as a possible toxic metabolite in the immunodeficiency associated with purine nucleoside phosphorylase deficiency. J Clin Invest. 1978 May;61(5):1405–1409. doi: 10.1172/JCI109058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. S., Hutton J. J. Micromethod for quantitation of adenosine deaminase activity in cells from human peripheral blood. Biochem Med. 1975 May;13(1):46–55. doi: 10.1016/0006-2944(75)90139-8. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Ammann A. J., Wara D. W., Sandman R., Diamond L. K. Nucleoside-phosphorylase deficiency in a child with severely defective T-cell immunity and normal B-cell immunity. Lancet. 1975 May 3;1(7914):1010–1013. doi: 10.1016/s0140-6736(75)91950-9. [DOI] [PubMed] [Google Scholar]
- Gmelig-Meyling F., Ballieux R. E. Simplified procedure for the separation of human T and non-T cells. Vox Sang. 1977 Jul;33(1):5–8. doi: 10.1111/j.1423-0410.1977.tb02229.x. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Brown G. Purification of human T and B lymphocytes. J Immunol. 1974 Jan;112(1):420–423. [PubMed] [Google Scholar]
- Hunninghake G. W., Haynes B. F., Parrillo J. E., Fauci A. S. Comparison of the relative cytotoxic effector cell capabilities and the proportions of cells bearing various surface markers in human tonsil and peripheral blood mononuclear cells. Clin Exp Immunol. 1978 Apr;32(1):186–191. [PMC free article] [PubMed] [Google Scholar]
- Ives D. H., Durham J. P., Tucker V. S. Rapid determination of nucleoside kinase and nucleotidase activities with tritium-labeled substrates. Anal Biochem. 1969 Apr 4;28(1):192–205. doi: 10.1016/0003-2697(69)90170-5. [DOI] [PubMed] [Google Scholar]
- Kaplan J., Shope T. C., Peterson W. D., Jr Epstein-barr virus-negative human malignant T-cell lines. J Exp Med. 1974 May 1;139(5):1070–1076. doi: 10.1084/jem.139.5.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minowada J., Onuma T., Moore G. E. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972 Sep;49(3):891–895. [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Nilsson K., Pontén J. Classification and biological nature of established human hematopoietic cell lines. Int J Cancer. 1975 Feb 15;15(2):321–341. doi: 10.1002/ijc.2910150217. [DOI] [PubMed] [Google Scholar]
- Ochs U. H., Chen S. H., Ochs H. D., Osborne W. R., Scott C. R. Purine nucleoside phosphorylase deficiency: a molecular model for selective loss of T cell function. J Immunol. 1979 Jun;122(6):2424–2429. [PubMed] [Google Scholar]
- Roos D., Loos J. A. Changes in the carbohydrate metabolism of mitogenically stimulated human peripheral lymphocytes. I. Stimulation by phytohaemagglutinin. Biochim Biophys Acta. 1970 Dec 29;222(3):565–582. doi: 10.1016/0304-4165(70)90182-0. [DOI] [PubMed] [Google Scholar]
- Rowe M., de Gast C. G., Platts-Mills T. A., Asherson G. L., Webster A. D., Johnson S. M. 5'-nucleotidase of B and T lymphocytes isolated from human peripheral blood. Clin Exp Immunol. 1979 Apr;36(1):97–101. [PMC free article] [PubMed] [Google Scholar]
- Stoop J. W., Zegers B. J., Hendrickx G. F., van Heukelom L. H., Staal G. E., de Bree P. K., Wadman S. K., Ballieux R. E. Purine nucleoside phosphorylase deficiency associated with selective cellular immunodeficiency. N Engl J Med. 1977 Mar 24;296(12):651–655. doi: 10.1056/NEJM197703242961203. [DOI] [PubMed] [Google Scholar]
- Webster A. D., North M., Allsop J., Asherson G. L., Watts R. W. Purine metabolism in lymphocytes from patients with primary hypogammaglobulinaemia. Clin Exp Immunol. 1978 Mar;31(3):456–463. [PMC free article] [PubMed] [Google Scholar]
- de Gast G. C., Platts-Mills T. A. Functional studies on lymphocytes in adult human bone marrow. II. Isolated surface IgM-positive cells. J Immunol. 1979 Jan;122(1):285–290. [PubMed] [Google Scholar]


