Abstract
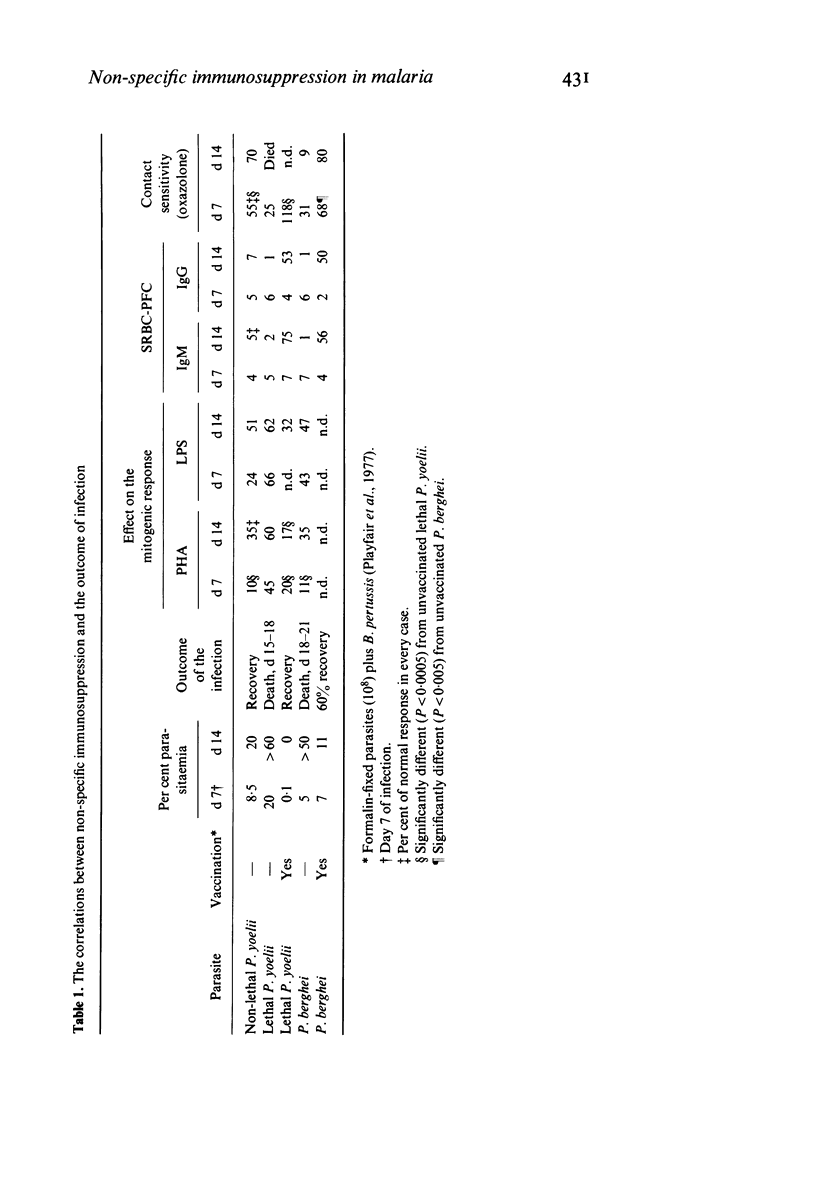
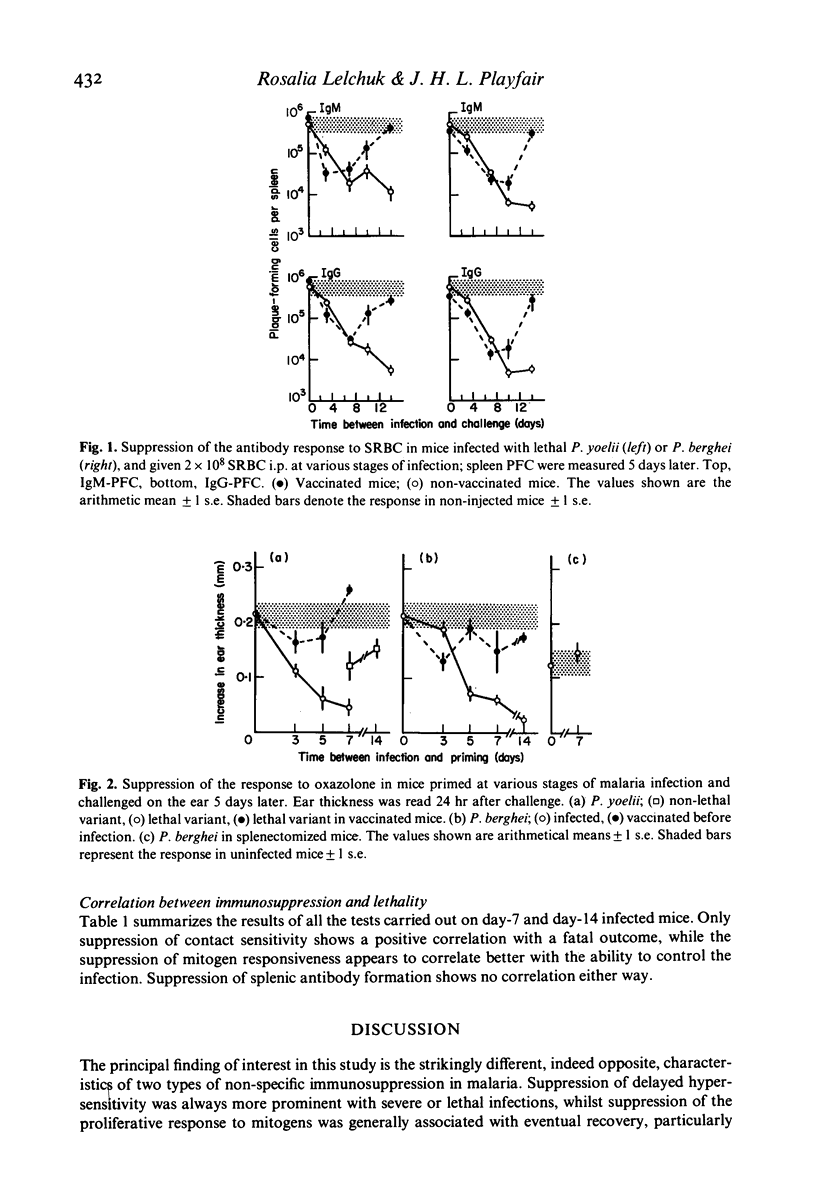
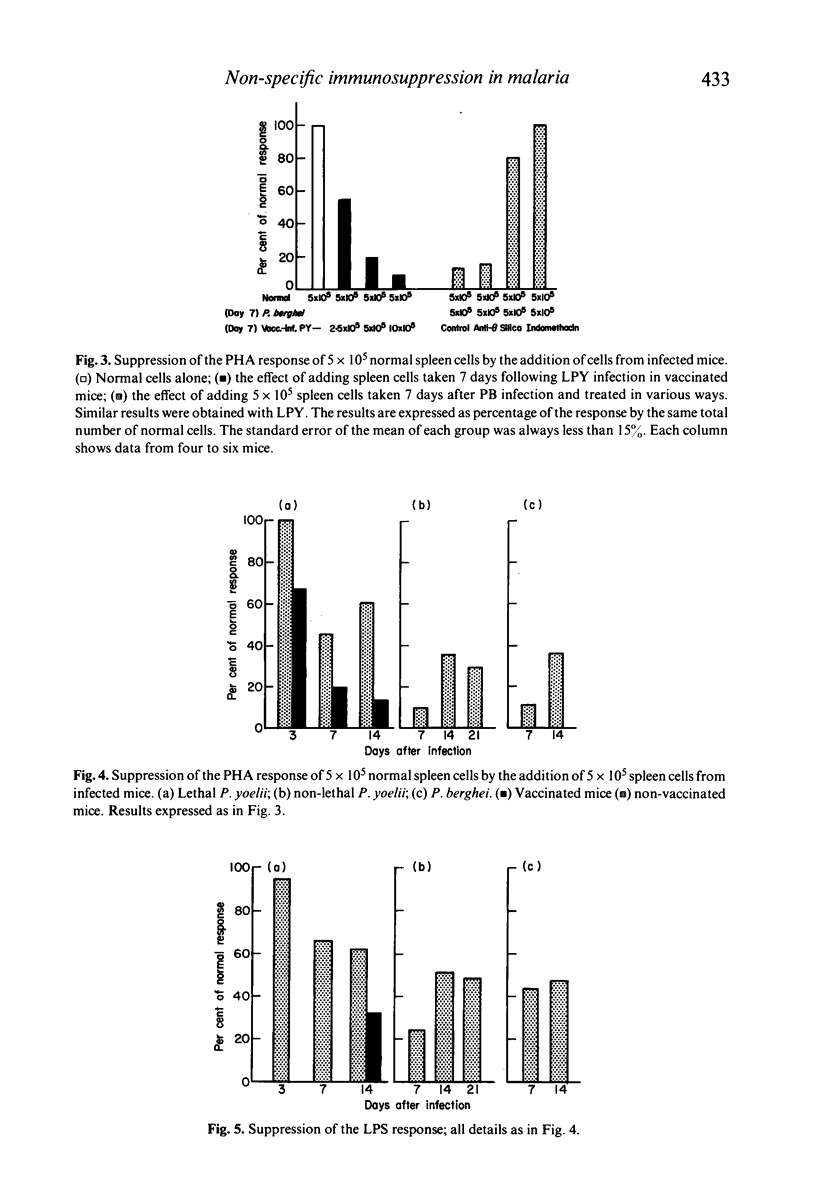
A comparative study of non-specific immunosuppression by malaria has been carried out in five situations: in both unvaccinated and vaccinated mice infected with the lethal Plasmodium yoelii or the lethal Plasmodium berghei, and in the unvaccinated non-lethal P. yoelii infection. Spleen cells showed a suppressive effect on the normal blastogenic response to mitogens. This suppression was strongest in the mice vaccinated before infection with the lethal P. yoelii and in those infected with non-lethal P. yoelii, suggesting that the suppressive effect did not interfere with recovery. Silica, anti-Thy-1, and indomethacin treatment suggested that this suppression was caused by macrophages. However, the plaque-forming cell response to sheep RBC in vivo was suppressed equally in every case at the peak of the parasitaemia, whereas the suppression of contact sensitivity to oxazolone was strongest in mice with fatal infections. We suggest that different suppressor mechanisms operate in malaria, some being harmful to the host and others possibly beneficial.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berenbaum M. C. Is azathioprine a better immunosuppressive than 6-mercaptopurine? Clin Exp Immunol. 1971 Jan;8(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- Colizzi V., Bozzi L. A mechanism for the depression of contact sensitivity with B-cell mitogens. Ann Immunol (Paris) 1979 Sep-Oct;130C(5):659–673. [PubMed] [Google Scholar]
- Cottrell B. J., Playfair J. H., De Souza B. J. Cell-mediated immunity in mice vaccinated against malaria. Clin Exp Immunol. 1978 Nov;34(2):147–158. [PMC free article] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Finerty J. F., Krehl E. P. Cyclophosphamide pretreatment and protection against malaria. Infect Immun. 1976 Oct;14(4):1103–1105. doi: 10.1128/iai.14.4.1103-1105.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood B. M. Possible role of a B-cell mitogen in hypergammaglobulinaemia in malaria and trypanosomiasis. Lancet. 1974 Mar 16;1(7855):435–436. doi: 10.1016/s0140-6736(74)92386-1. [DOI] [PubMed] [Google Scholar]
- Grimm W., Seitz M., Kirchner H., Gemsa D. Prostaglandin synthesis in spleen cell cultures of mice injected with Corynebacterium parvum. Cell Immunol. 1978 Oct;40(2):419–426. doi: 10.1016/0008-8749(78)90349-0. [DOI] [PubMed] [Google Scholar]
- Jayawardena A. N., Targett G. A., Leuchars E., Carter R. L., Doenhoff M. J., Davies A. J. T-cell activation in murine malaria. Nature. 1975 Nov 13;258(5531):149–151. doi: 10.1038/258149a0. [DOI] [PubMed] [Google Scholar]
- Lee S. K., Singh J., Taylor R. B. Subclasses of T cells with different sensitivities to cytotoxic antibody in the presence of anesthetics. Eur J Immunol. 1975 Apr;5(4):259–262. doi: 10.1002/eji.1830050408. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Dhaliwal S. S., Teh K. L. Dissociative effects of malarial infection on humoral and cell-mediated immunity in mice. Immunology. 1979 May;37(1):35–44. [PMC free article] [PubMed] [Google Scholar]
- Michel J. C., Lagrange P. H., Hurtrel B. Modulation by malaria infection of the induction of T lymphocyte-dependent delayed-type hypersensitivity and antibody formation to sheep erythrocytes in mice. Parasite Immunol. 1979 Winter;1(4):267–275. doi: 10.1111/j.1365-3024.1979.tb00712.x. [DOI] [PubMed] [Google Scholar]
- Playfair J. H., De Souza J. B., Dockrell H. M., Agomo P. U., Taverne J. Cell-mediated immunity in the liver of mice vaccinated against malaria. Nature. 1979 Dec 13;282(5740):731–734. doi: 10.1038/282731a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg Y. J. Autoimmune and polyclonal B cell responses during murine malaria. Nature. 1978 Jul 13;274(5667):170–172. doi: 10.1038/274170a0. [DOI] [PubMed] [Google Scholar]
- Scott M. T. Depression of delayed-type hypersensitivity by Corynebacterium parvum: mandatory role of the spleen. Cell Immunol. 1974 Aug;13(2):251–263. doi: 10.1016/0008-8749(74)90243-3. [DOI] [PubMed] [Google Scholar]
- Spira D. T., Golenser J., Gery I. The reactivity of spleen cells from malarious rats to non-specific mitogens. Clin Exp Immunol. 1976 Apr;24(1):139–145. [PMC free article] [PubMed] [Google Scholar]
- Sy M. S., Miller S. D., Kowach H. B., Claman H. N. A splenic requirement for the generation of suppressor T cells. J Immunol. 1977 Dec;119(6):2095–2099. [PubMed] [Google Scholar]
- Warren H. S., Weidanz W. P. Malarial immunodepression in vitro: adherent spleen cells are functionally defective as accessory cells in the response to horse erythrocytes. Eur J Immunol. 1976 Nov;6(11):816–819. doi: 10.1002/eji.1830061112. [DOI] [PubMed] [Google Scholar]
- Weinbaum F. I., Weintraub J., Nkrumah F. K., Evans C. B., Tigelaar R. E., Rosenberg Y. J. Immunity to Plasmodium berghei yoelii in mice. II. Specific and nonspecific cellular and humoral responses during the course of infection. J Immunol. 1978 Aug;121(2):629–636. [PubMed] [Google Scholar]
- Wiedanz W. P., Rank R. G. Regional immunosuppression induced by Plasmodium berghei yoelii infection in mice. Infect Immun. 1975 Jan;11(1):211–212. doi: 10.1128/iai.11.1.211-212.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson W. A., Greenwood B. M. Impairment of the immune response to vaccination after acute malaria. Lancet. 1978 Jun 24;1(8078):1328–1329. doi: 10.1016/s0140-6736(78)92403-0. [DOI] [PubMed] [Google Scholar]
- Wyler D. J., Oppenheim J. J., Koontz L. C. Influence of malaria infection on the elaboration of soluble mediators by adherent mononuclear cells. Infect Immun. 1979 Apr;24(1):151–159. doi: 10.1128/iai.24.1.151-159.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zembala M., Asherson G. L., Noworolski J., Mayhew B. Contact sensitivity to picryl chloride: the occurrence of B suppressor cells in the lymph nodes and spleen of immunized mice. Cell Immunol. 1976 Aug;25(2):266–278. doi: 10.1016/0008-8749(76)90117-9. [DOI] [PubMed] [Google Scholar]


