Abstract
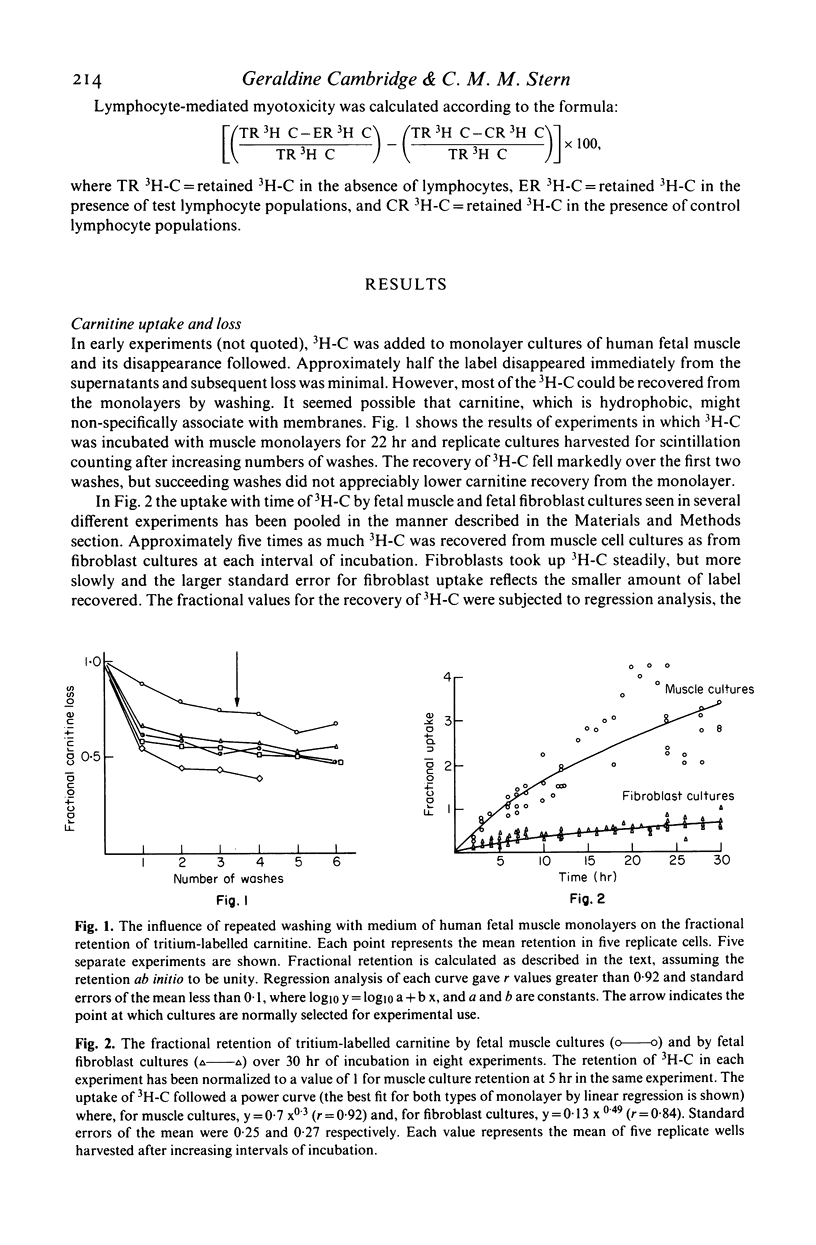
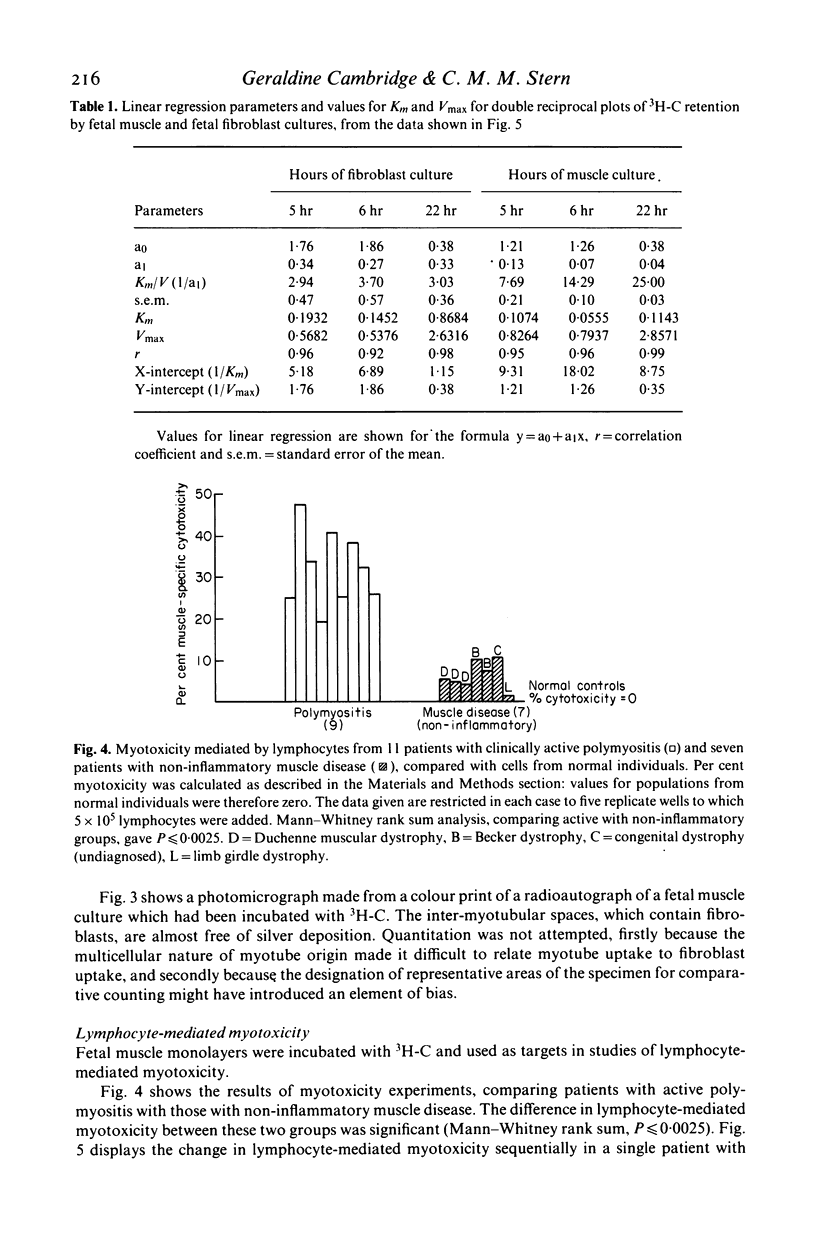
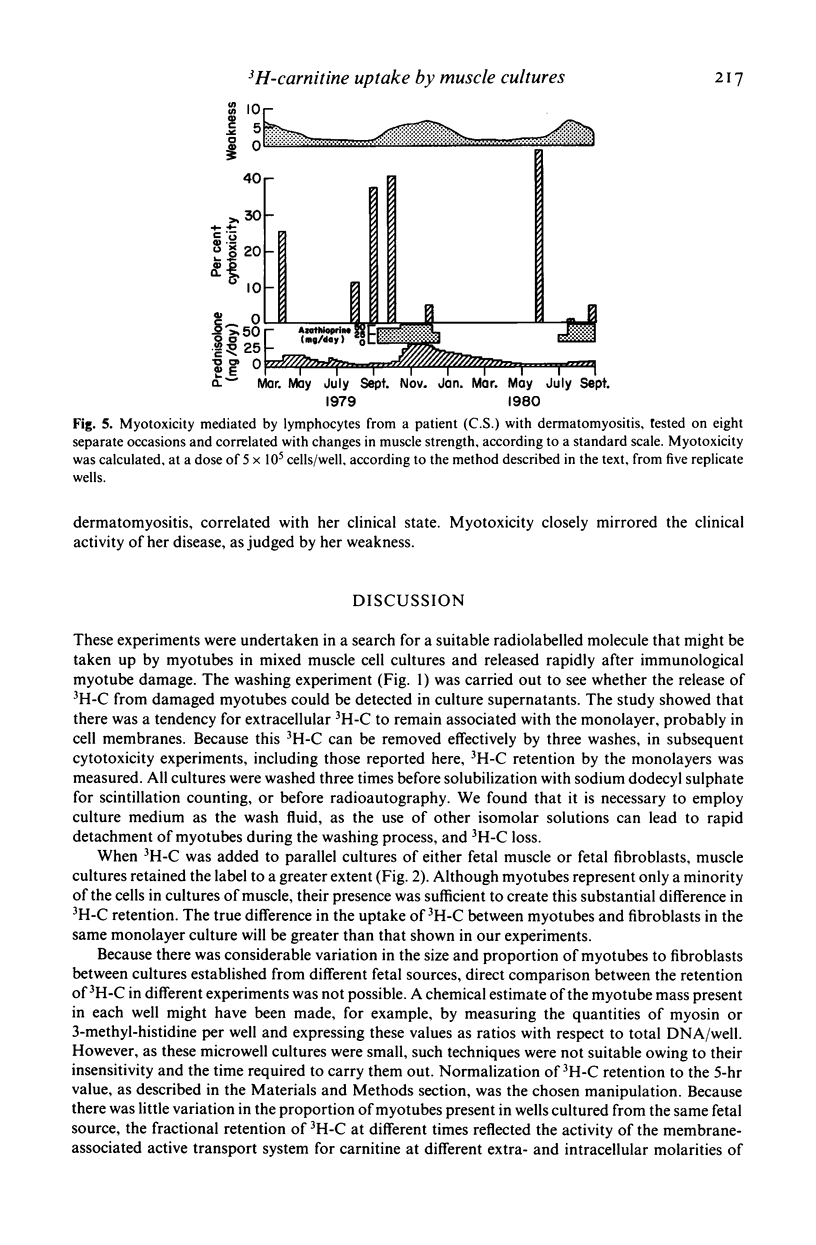
As a novel approach to the investigation of immune responses directed against muscle antigens in inflammatory muscle disease, the use of tritium-labelled carnitine as a selective marker for myotubes in monolayer cultures was investigated. Tritium-labelled carnitine was incubated either with monolayer cultures of human fetal muscle (which contain fibroblasts and myotubes) or with syngeneic monolayer cultures of human fetal fibroblasts. The rate of uptake and loss of tritium-labelled carnitine by muscle cultures was compared with that shown by fibroblast cultures; uptake being five times greater for muscle. Values for Km and Vmax were derived for both tissues in culture, the ratio Km/Vmax being 3·1 for muscle cultures and 0·46 for fibroblast cultures, indicating the presence of the active transport system for carnitine in the myotube membrane. Freeze-dried radioautographs of muscle monolayers, previously incubated with tritium-labelled carnitine, were made and confirmed the specific intra-tubular localization of the label. Fetal muscle monolayers, previously incubated with tritium-labelled carnitine, were used as targets in long-term cytotoxicity experiments into lymphocyte-mediated myotoxicity. Peripheral blood lymphocytes from patients with inflammatory muscle disease were shown to be myotoxic, but lymphocytes from normal individuals or those with non-inflammatory muscle disease were not. This system is likely to prove much more sensitive than those methods employing chromium-51-labelled cultures. Carnitine-based measures of myotoxicity closely followed the clinical activity of the disease in sequential studies carried out on one patient and the test shows considerable potential as a means of assessing myotube killing by lymphocytes on a per-cell basis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böhmer T., Eiklid K., Jonsen J. Carnitine uptake into human heart cells in culture. Biochim Biophys Acta. 1977 Mar 17;465(3):627–633. doi: 10.1016/0005-2736(77)90278-4. [DOI] [PubMed] [Google Scholar]
- Currie S. Destruction of muscle cultures by lymphocytes from cases of polymyositis. Acta Neuropathol. 1970;15(1):11–19. doi: 10.1007/BF00690685. [DOI] [PubMed] [Google Scholar]
- Dawkins R. L., Mastaglia F. L. Cell-mediated cytotoxicity to muscle in polymyositis. Effect of immunosuppression. N Engl J Med. 1973 Mar 1;288(9):434–438. doi: 10.1056/NEJM197303012880903. [DOI] [PubMed] [Google Scholar]
- Friedlander M., Beyer E. C., Fischman D. A. Muscle development in vitro following X irradiation. Dev Biol. 1978 Oct;66(2):457–469. doi: 10.1016/0012-1606(78)90251-8. [DOI] [PubMed] [Google Scholar]
- Fritz I. B., Marquis N. R. The role of acylcarnitine esters and carnitine palmityltransferase in the transport of fatty acyl groups across mitochondrial membranes. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1226–1233. doi: 10.1073/pnas.54.4.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greville G. D., Tubbs P. K. The catabolism of long chain fatty acids in mammalian tissues. Essays Biochem. 1968;4:155–212. [PubMed] [Google Scholar]
- Moss M., Norris J. S., Peck E. J., Jr, Schwartz R. J. Alterations in iodinated cell surface proteins during myogenesis. Exp Cell Res. 1978 May;113(2):445–450. doi: 10.1016/0014-4827(78)90388-9. [DOI] [PubMed] [Google Scholar]
- Partridge T. A., Smith P. D. A quantitative test to detect lymphocytes sensitized against the surface of muscle cells. Clin Exp Immunol. 1976 Jul;25(1):139–143. [PMC free article] [PubMed] [Google Scholar]
- Pearson C. M., Bohan A. The spectrum of polymyositis and dermatomyositis. Med Clin North Am. 1977 Mar;61(2):439–457. doi: 10.1016/s0025-7125(16)31343-8. [DOI] [PubMed] [Google Scholar]
- Rebouche C. J. Carnitine movement across muscle cell membranes. Studies in isolated rat muscle. Biochim Biophys Acta. 1977 Nov 15;471(1):145–155. doi: 10.1016/0005-2736(77)90402-3. [DOI] [PubMed] [Google Scholar]
- Willner J. H., Ginsburg S., Dimauro S. Active transport of carnitine into skeletal muscle. Neurology. 1978 Jul;28(7):721–724. doi: 10.1212/wnl.28.7.721. [DOI] [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]



