Abstract
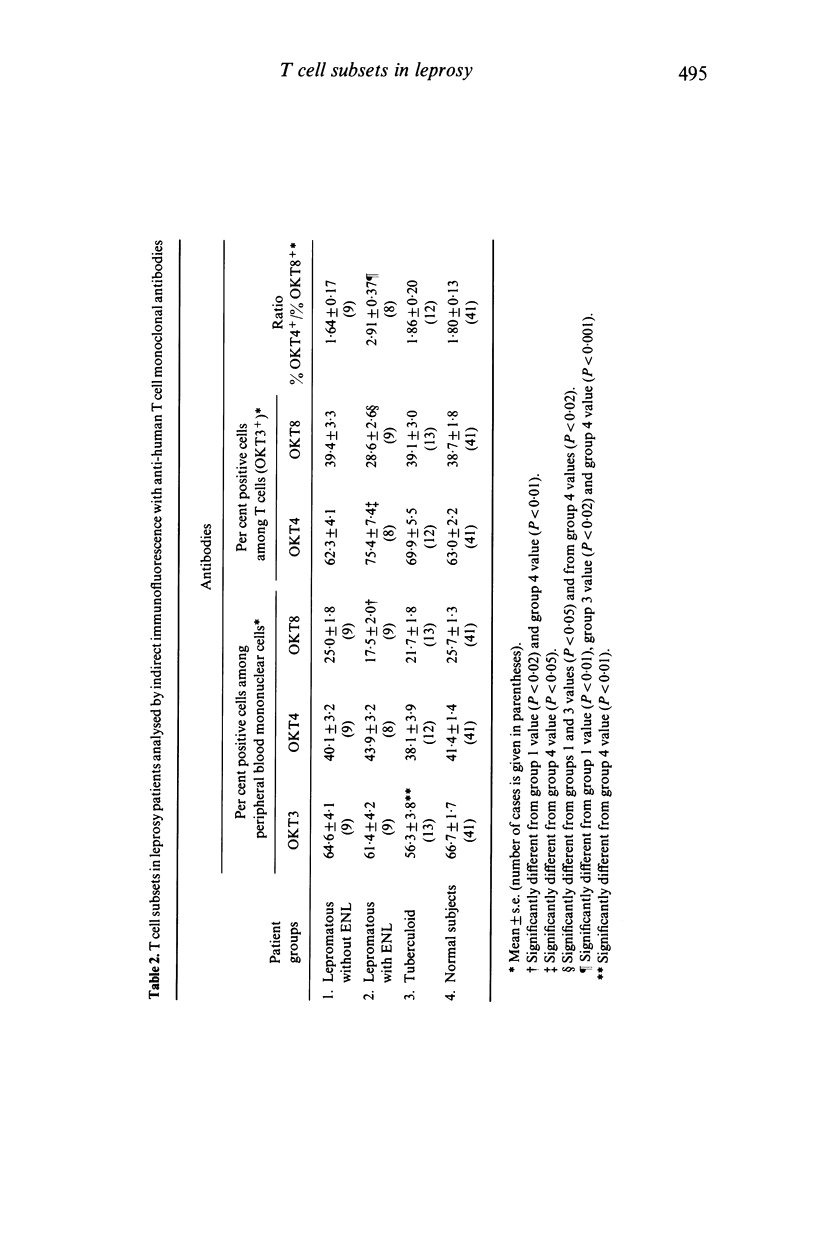
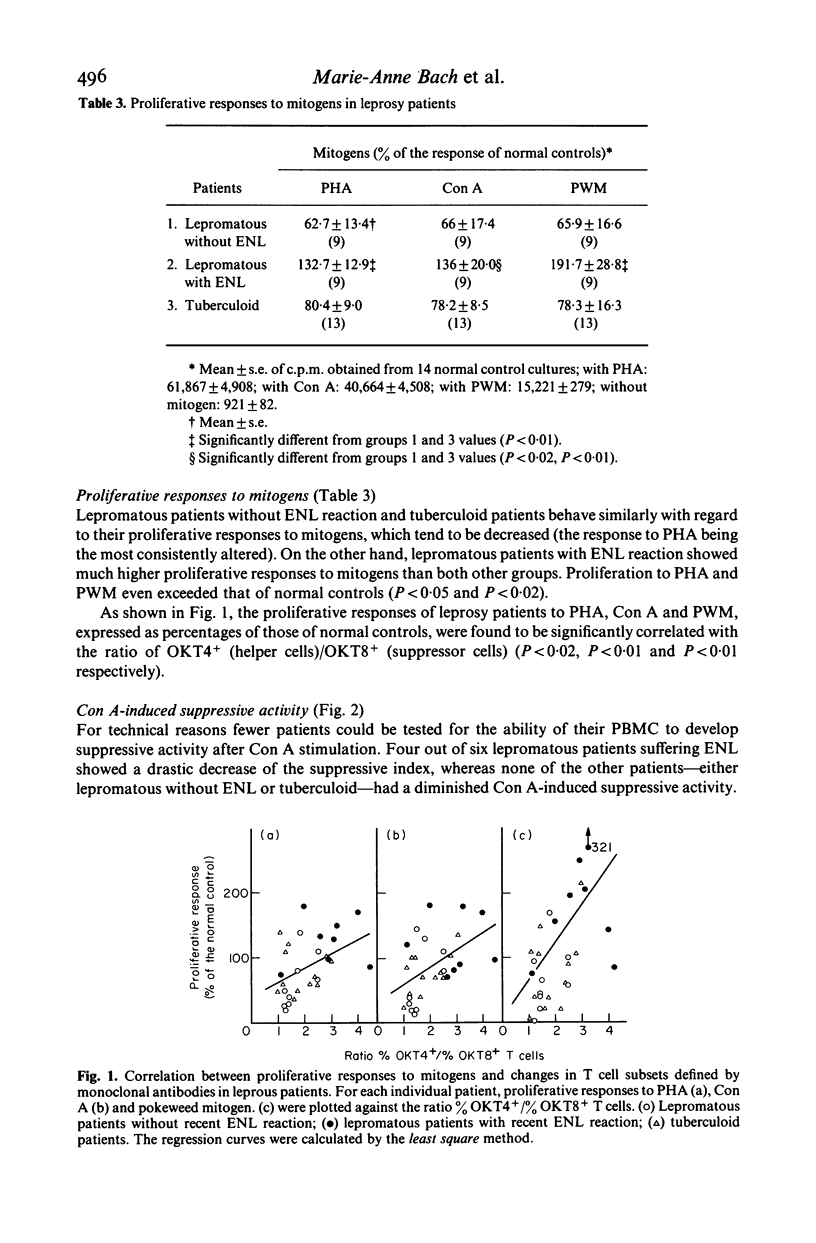
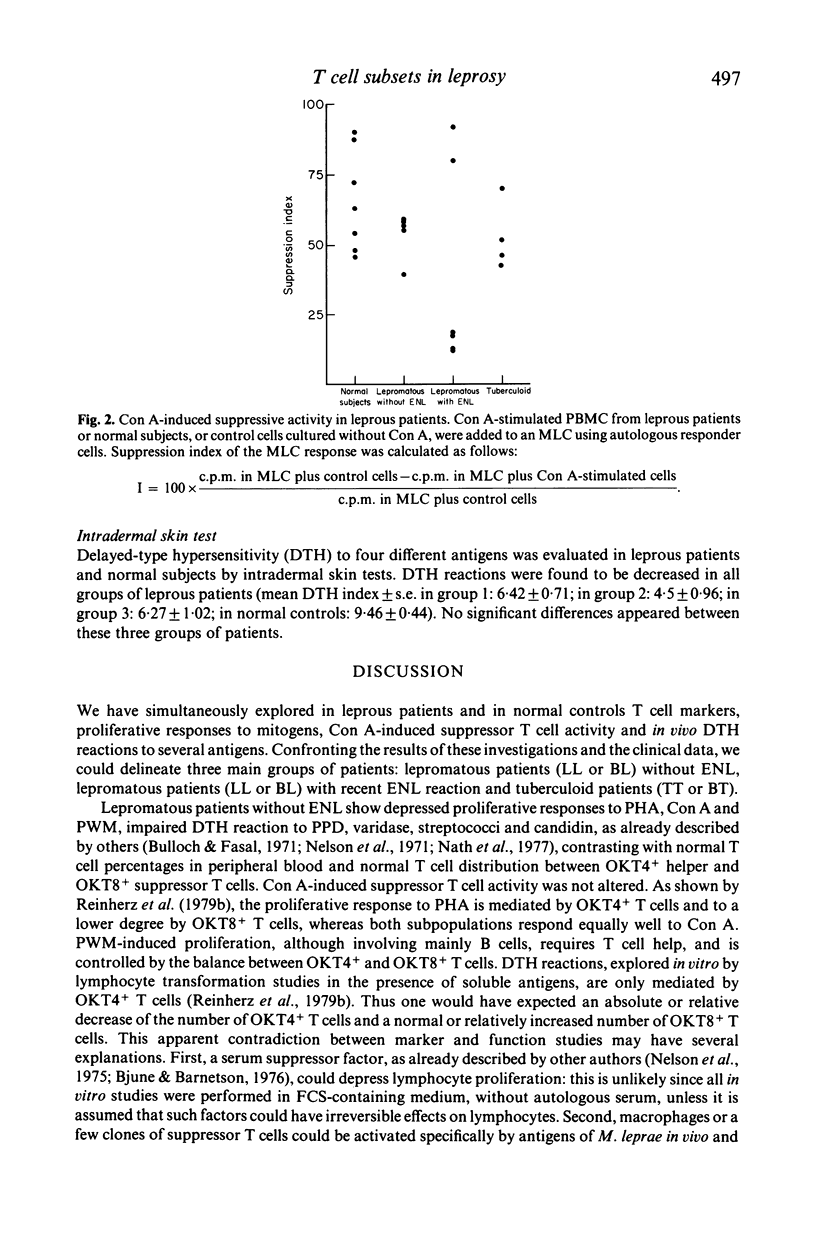
T cell subsets and T cell functions were explored in 31 leprosy patients with the following methods: determination of the percentages of the different T cell subpopulations defined by monoclonal antibodies directed at total T cells, helper T cells and suppressor/cytotoxic T cells; measurement of the in vitro proliferative responses to mitogens; study of the concanavalin A-induced suppressive activity, assessed on MLC; measurement of delayed-type hypersensitivity by skin testing. The confrontation between immunological lepromatous patients without type-2 reaction (erythema nodosum leprosum), (2) lepromatous patients without ENL (erythema nodosum leprosum), (2) lepromatous patients was recent ENL and (3) tuberculoid patients. Unexpectedly, groups 1 and 3, although differing strongly in their clinical status and their sensitivity to lepromin (absent in group 1 and strong in group 3), showed a similar immunological profile with a normal percentage of T cells and a normal distribution of T cells among the major T cell subset contrasting with a moderate decrease of proliferative responses to mitogens and impaired delayed-type hypersensitivity reactions. Concanavalin A-induced suppressive activity was type-2 reaction) strongly differed from both other groups, showing striking abnormalities other groups, showing striking abnormalities of the repartition of the T cell subsets, with increased percentages of helper T cells and decreased percentages of suppressor T cells, and elevated proliferative responses to mitogens. Concanavalin A-induced suppressive activity was reduced in most patients of this group. It is suggested that this imbalance between T cell subsets contributes to the occurrence of ENL reactions in lepromatous patients.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders E. M., McAdam K. P., Anders R. F. Cell-mediated immunity in amyloidosis secondary to lepromatous leprosy. Clin Exp Immunol. 1977 Jan;27(1):111–117. [PMC free article] [PubMed] [Google Scholar]
- Bjorvatn B., Barnetson R. S., Kronvall G., Zubler R. H., Lambert P. H. Immune complexes and complement hypercatabolism in patients with leprosy. Clin Exp Immunol. 1976 Dec;26(3):388–396. [PMC free article] [PubMed] [Google Scholar]
- Bjune G., Barnetson R. S. Plasma factors in delayed-type hypersensitivity. Augmentation of lymphocyte responses in borderline leprosy reactions. Clin Exp Immunol. 1976 Dec;26(3):397–402. [PMC free article] [PubMed] [Google Scholar]
- Bjune G. In vitro lymphocyte stimulation in leprosy; simultaneous stimulation with Mycobacterium leprae antigens and phytohaemagglutinin. Clin Exp Immunol. 1979 Jun;36(3):479–487. [PMC free article] [PubMed] [Google Scholar]
- Bullock W. E., Carlson E. M., Gershon R. K. The evolution of immunosuppressive cell populations in experimental mycobacterial infection. J Immunol. 1978 May;120(5):1709–1716. [PubMed] [Google Scholar]
- Bullock W. E., Jr, Fasal P. Studies of immune mechanisms in leprosy. 3. The role of cellular and humoral factors in impairment of the in vitro immune response. J Immunol. 1971 Apr;106(4):888–899. [PubMed] [Google Scholar]
- Charpentier B., Carnaud C., Bach J. F. Selective depression of the xenogeneic cell-mediated lympholysis in systemic lupus erythematosus. J Clin Invest. 1979 Aug;64(2):351–360. doi: 10.1172/JCI109469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godal T. Immunological aspects of leprosy--present status. Prog Allergy. 1978;25:211–242. [PubMed] [Google Scholar]
- Han S. H., Weiser R. S., Kau S. T. Prolonged survival of skin allografts in leprosy patients. Int J Lepr Other Mycobact Dis. 1971 Jan-Mar;39(1):1–6. [PubMed] [Google Scholar]
- Lim S. D., Jacobson R. R., Park B. H., Good R. A. Leprosy XII. Quantitative analysis of thymus-derived lymphocyte response to phytohemagglutinin in leprosy. Int J Lepr Other Mycobact Dis. 1975 Apr-Jun;43(2):95–100. [PubMed] [Google Scholar]
- Mehra V., Mason L. H., Fields J. P., Bloom B. R. Lepromin-induced suppressor cells in patients with leprosy. J Immunol. 1979 Oct;123(4):1813–1817. [PubMed] [Google Scholar]
- Nath I., Curtis J., Sharma A. K., Talwar G. P. Circulating T-cell numbers and their mitogenic potential in leprosy--correlation with mycobacterial load. Clin Exp Immunol. 1977 Sep;29(3):393–400. [PMC free article] [PubMed] [Google Scholar]
- Nath I., Narayanan R. B., Mehra N. K., Sharma A. K., Gupte M. D. Concanavalin A induced suppressor activity in human leprosy. J Clin Lab Immunol. 1979 Nov;2(4):319–324. [PubMed] [Google Scholar]
- Nelson D. S., Penrose J. M., Waters M. F., Pearson J. M., Nelson M. Depressive effect of serum from patients with leprosy on mixed lymphocyte reactions. Influence of anti-leprosy treatment. Clin Exp Immunol. 1975 Dec;22(3):385–392. [PMC free article] [PubMed] [Google Scholar]
- Rea T. H., Levan N. E. Variations in dinitrochlorobenzene responsivity in untreated leprosy: evidence of a beneficial role for anergy. Int J Lepr Other Mycobact Dis. 1980 Jun;48(2):120–125. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with the human cytotoxic/suppressor T cell subset previously defined by a heteroantiserum termed TH2. J Immunol. 1980 Mar;124(3):1301–1307. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody with selective reactivity with functionally mature human thymocytes and all peripheral human T cells. J Immunol. 1979 Sep;123(3):1312–1317. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4061–4065. doi: 10.1073/pnas.76.8.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Ridley D. S., Hilson G. R. A logarithmic index of bacilli in biopsies. I. Method. Int J Lepr Other Mycobact Dis. 1967 Apr-Jun;35(2):184–186. [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- Rojas-Espinosa O., Mendez-Navarrete I., Estrada-Parra S. Presence of C1q-reactive immune complexes in patients with leprosy. Clin Exp Immunol. 1972 Oct;12(2):215–223. [PMC free article] [PubMed] [Google Scholar]
- Sakane T., Green I. Human suppressor T cells induced by concanavalin A: suppressor T cells belong to distinctive T cell subclasses. J Immunol. 1977 Sep;119(3):1169–1178. [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Green I. Studies of immune functions of patients with systemic lupus erythematosus. I. Dysfunction of suppressor T-cell activity related to impaired generation of, rather than response to, suppressor cells. Arthritis Rheum. 1978 Jul-Aug;21(6):657–664. doi: 10.1002/art.1780210608. [DOI] [PubMed] [Google Scholar]
- Turcotte R. Suppressor cells in experimental murine leprosy. Int J Lepr Other Mycobact Dis. 1978 Jul-Dec;46(3-4):358–363. [PubMed] [Google Scholar]
- Turk J. L., Bryceson A. D. Immunological phenomena in leprosy and related diseases. Adv Immunol. 1971;13:209–266. doi: 10.1016/s0065-2776(08)60185-6. [DOI] [PubMed] [Google Scholar]
- Turk J. L. Leprosy as a model of subacute and chronic immunologic diseases. J Invest Dermatol. 1976 Sep;67(3):457–463. doi: 10.1111/1523-1747.ep12514734. [DOI] [PubMed] [Google Scholar]
- Waldorf D. S., Sheagren J. N., Trautman J. R., Block J. B. Impaired delayed hypersensitivity in patients with lepromatous leprosy. Lancet. 1966 Oct 8;2(7467):773–776. doi: 10.1016/s0140-6736(66)90366-7. [DOI] [PubMed] [Google Scholar]
- Wemambu S. N., Turk J. L., Waters M. F., Rees R. J. Erythema nodosum leprosum: a clinical manifestation of the arthus phenomenon. Lancet. 1969 Nov 1;2(7627):933–935. doi: 10.1016/s0140-6736(69)90592-3. [DOI] [PubMed] [Google Scholar]
- Winchester R. J., Fu S. M., Hoffman T., Kunkel H. G. IgG on lymphocyte surfaces; technical problems and the significance of a third cell population. J Immunol. 1975 Apr;114(4):1210–1212. [PubMed] [Google Scholar]


