Abstract
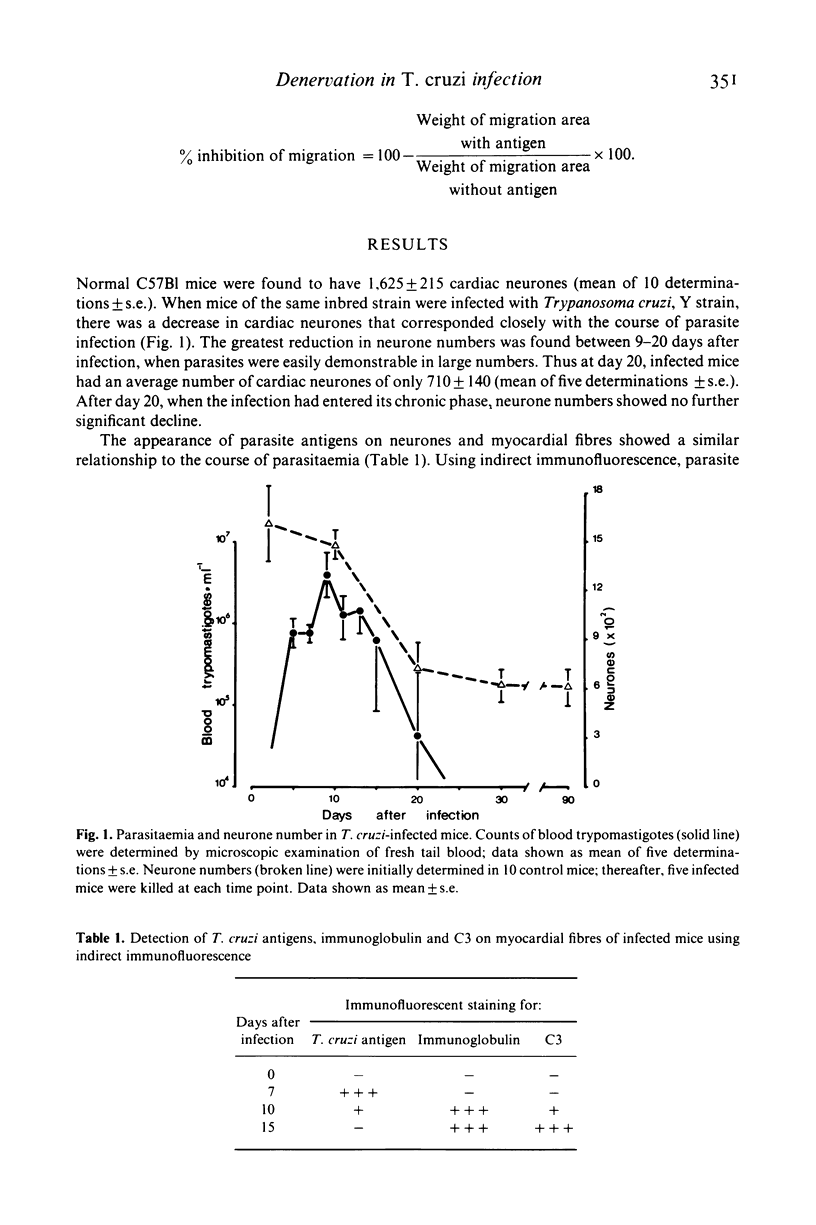
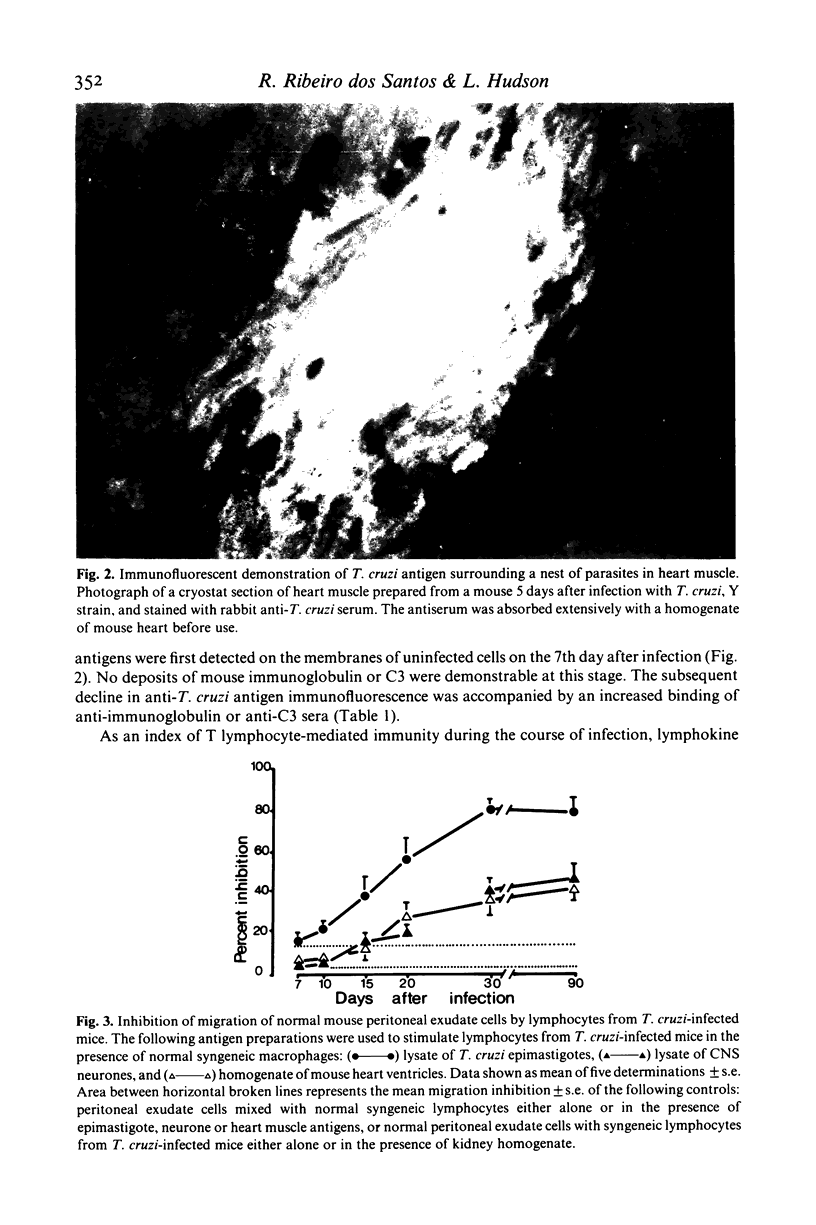
C57B1 mice were infected with Trypanosoma cruzi Y strain trypomastigotes and showed a peak of parasitaemia 9 days after infection. Virtually all mice survived the acute stage of infection and were aparasitaemic thereafter. Coincident with the peak of parasitaemia, there was a progressive loss of cardiac neurones (up to the 20th day after infection) and an appearance of T. cruzi antigen on myofibres. Anti-parasite immunity, evidenced by a significant inhibition of macrophage migration in the presence of T. cruzi antigens (MIF test) and the deposition of complement and immunoglobulin in vivo around the nests of parasites, developed between days 7-10 after infection. Immunity to "self' components (MIF) test using neurone and heart antigens) was not detected until 15-20 days after infection. Although the MIF test detected a progressive increase in anti-neurone immunity between 20-90 days after infection, there was no additional loss of cardiac neurones during this period. In contrast to current hypotheses, these data suggest that the immunity to heart and neuronal antigens commonly detected in animals infected with T. cruzi is the result rather than the cause, of host cell destruction.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bøyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol. 1976 Jun;Suppl 5:9–15. [PubMed] [Google Scholar]
- DAVID J. R., AL-ASKARI S., LAWRENCE H. S., THOMAS L. DELAYED HYPERSENSITIVITY IN VITRO. I. THE SPECIFICITY OF INHIBITION OF CELL MIGRATION BY ANTIGENS. J Immunol. 1964 Aug;93:264–273. [PubMed] [Google Scholar]
- GEORGE M., VAUGHAN J. H. In vitro cell migration as a model for delayed hypersensitivity. Proc Soc Exp Biol Med. 1962 Nov;111:514–521. doi: 10.3181/00379727-111-27841. [DOI] [PubMed] [Google Scholar]
- KOEBERLE F. ENTEROMEGALY AND CARDIOMEGALY IN CHAGAS DISEASE. Gut. 1963 Dec;4:399–405. doi: 10.1136/gut.4.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köberle F. The causation and importance of nervous lesions in American trypanosomiasis. Bull World Health Organ. 1970;42(5):739–743. [PMC free article] [PubMed] [Google Scholar]
- Santos-Buch C. A., Teixeira A. R. The immunology of experimental Chagas' disease. 3. Rejection of allogeneic heart cells in vitro. J Exp Med. 1974 Jul 1;140(1):38–53. doi: 10.1084/jem.140.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira A. R. Chagas' disease: trends in immunological research and prospects for immunoprophylaxis. Bull World Health Organ. 1979;57(5):697–710. [PMC free article] [PubMed] [Google Scholar]
- Teixeira A. R., Santos-Buch C. A. The immunology of experimental Chagas' disease. I. Preparation of Trypanosoma cruzi antigens and humoral antibody response to there antigens. J Immunol. 1974 Sep;113(3):859–869. [PubMed] [Google Scholar]
- Teixeira A. R., Teixeira M. L., Santos-Buch C. A. The immunology of experimental Chagas' disease. IV. Production of lesions in rabbits similar to those of chronic Chagas' disease in man. Am J Pathol. 1975 Jul;80(1):163–180. [PMC free article] [PubMed] [Google Scholar]