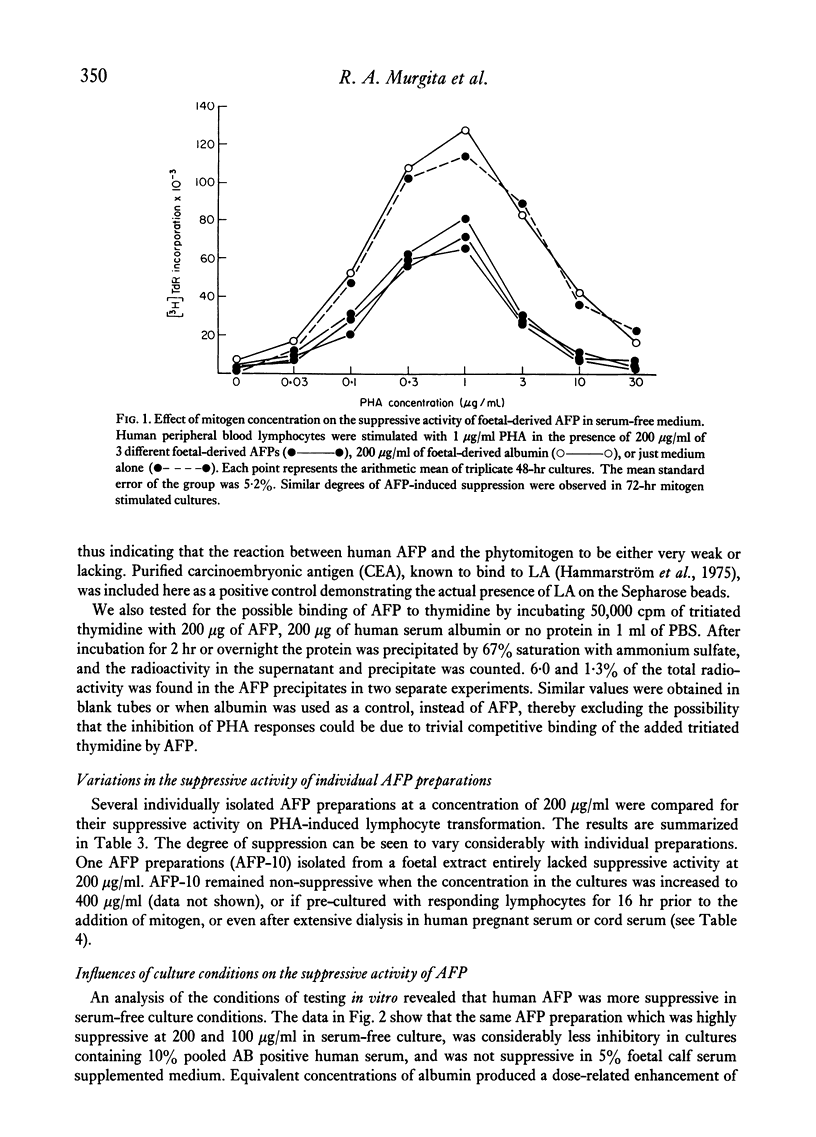
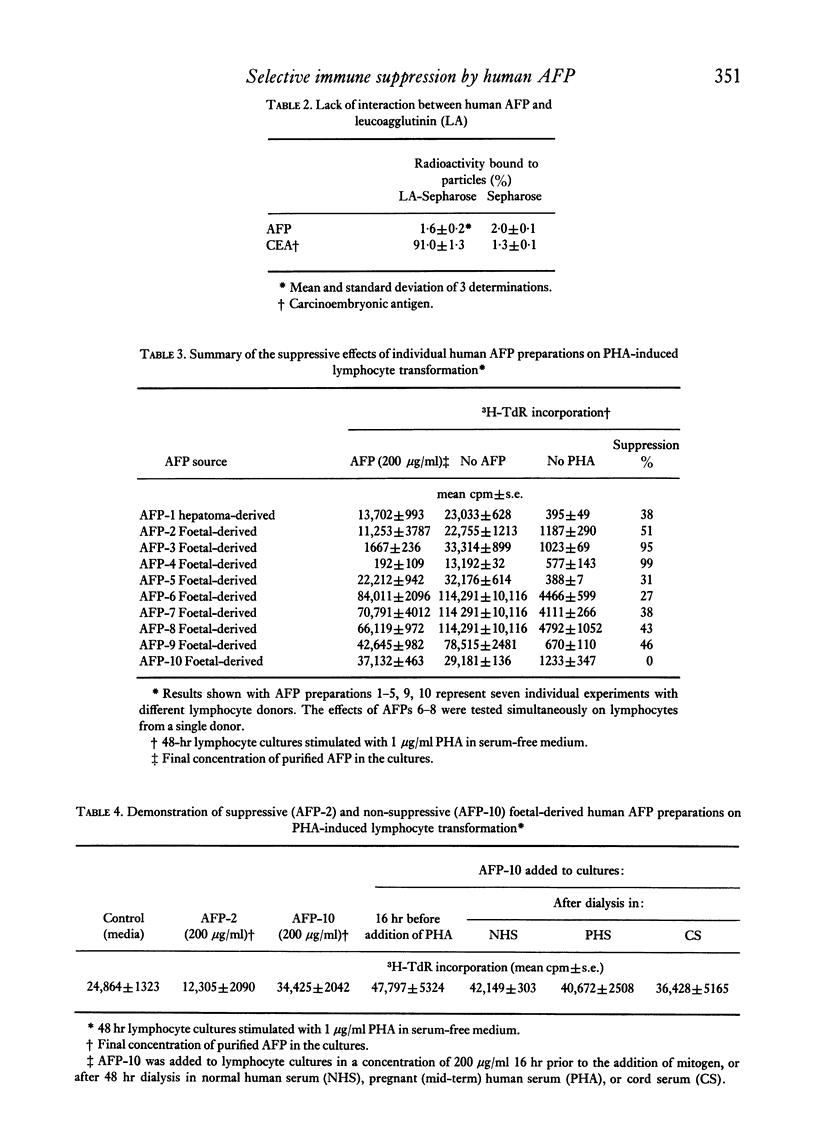
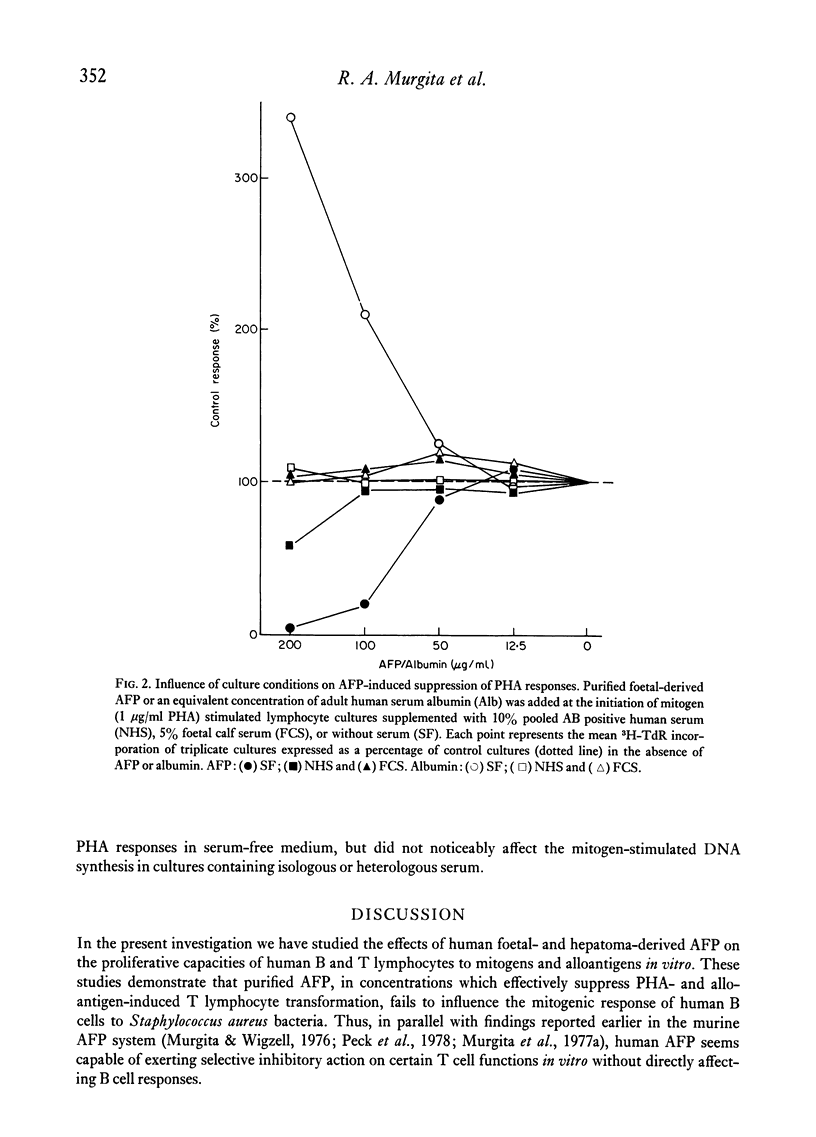
Abstract
Purified human alpha-foetoprotein (AFP) isolated from extracts of foetal and hepatoma tissues, and from cord serum was evaluated as to its suppressive effects on in vitro lymphocyte responses to stimuli which selectively trigger human B or T cells. The effects of equivalent concentrations of individual AFP preparations were compared on lymphocyte cultures stimulated with the human B cell mitogen Staphylococcus aureus strain Cowan I organisms, with the T cell mitogen phytohaemagglutinin (PHA), and with irradiated allogeneic lymphocytes in the one-way mixed lymphocyte reaction (MLR). PHA responses were significantly inhibited by most purified preparations of AFP in a dose-dependent manner, within the concentration range of 300 to 18 μg/ml. However, individual foetal-derived AFP preparations did vary in suppressive potency on PHA responses, and attempts to reactivate an inactive AFP were unsuccessful. In parallel cultures the mitogenic response to protein A expressing Staph. aureus bacteria was normal or even slightly enhanced by AFP. The one-way MLR was effectively suppressed at higher concentrations of AFP (300–600 μg/ml) than were required for inhibition of PHA responses. The inhibitory effect of AFP on PHA-induced lymphocyte proliferation was not altered by increases in the mitogen dose. No evidence was found that AFP merely inhibits PHA responses by direct interference with mitogen or by competition for cell surface receptors with the mitogen. The results reported here indicate that human AFP effectively suppresses certain T cell-mediated reactions, but not B cell responses in vitro, and these are in line with previously reported findings in the murine AFP system.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelev G. I. Alpha-fetoprotein as a marker of embryo-specific differentiations in normal and tumor tissues. Transplant Rev. 1974;20(0):3–37. doi: 10.1111/j.1600-065x.1974.tb00139.x. [DOI] [PubMed] [Google Scholar]
- Abelev G. I. Alpha-fetoprotein in ontogenesis and its association with malignant tumors. Adv Cancer Res. 1971;14:295–358. doi: 10.1016/s0065-230x(08)60523-0. [DOI] [PubMed] [Google Scholar]
- Adinolfi A., Adinolfi M., Lessof Alpha-feto-protein during development and in disease. J Med Genet. 1975 Jun;12(2):138–151. doi: 10.1136/jmg.12.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander P. Foetal "antigens" in cancer. Nature. 1972 Jan 21;235(5334):137–140. doi: 10.1038/235137a0. [DOI] [PubMed] [Google Scholar]
- Andersson L. C., Häyry P. Clonal isolation of alloantigen-reactive T-cells and characterization of their memory functions. Transplant Rev. 1975;25:121–162. doi: 10.1111/j.1600-065x.1975.tb00728.x. [DOI] [PubMed] [Google Scholar]
- Auer I. O., Kress H. G. Suppression of the primary cell-mediated immune response by human alpha1-fetoprotein in vitro. Cell Immunol. 1977 Apr;30(1):173–179. doi: 10.1016/0008-8749(77)90058-2. [DOI] [PubMed] [Google Scholar]
- Colley D. G., Wu A. Y., Waksman B. H. Cellular differentiation in the thymus. 3. Surface properties of rat thymus and lymph node cells separated on density gradients. J Exp Med. 1970 Dec 1;132(6):1107–1121. doi: 10.1084/jem.132.6.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren A., Svedjelund A., Wigzell H. Lymphocyte stimulation by protein A of Staphylococcus aureus. Eur J Immunol. 1976 Mar;6(3):207–213. doi: 10.1002/eji.1830060312. [DOI] [PubMed] [Google Scholar]
- Fuks A., Banjo C., Shuster J., Freedman S. O., Gold P. Carcinoembryonic antigen (CEA): molecular biology and clinical significance. Biochim Biophys Acta. 1975 Jul 11;417(2):123–152. doi: 10.1016/0304-419x(75)90002-5. [DOI] [PubMed] [Google Scholar]
- Gupta S., Good R. A. Alpha-fetoprotein and human lymphocyte subpopulations. J Immunol. 1977 Feb;118(2):405–408. [PubMed] [Google Scholar]
- Gustine D. L., Zimmerman E. F. Developmental changes in microheterogeneity of foetal plasma glycoproteins of mice. Biochem J. 1973 Mar;132(3):541–551. doi: 10.1042/bj1320541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarström S., Engvall E., Johansson B. G., Svensson S., Sundblad G., Goldstein I. J. Nature of the tumor-associated determinant(s) of carcinoembryonic antigen. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1528–1532. doi: 10.1073/pnas.72.4.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson S. G., Kjessler B., Sherman M. S. Determination of alpha-fetoprotein by a new paper disc radioimmunoassay of the sandwich type. Int Arch Allergy Appl Immunol. 1976;52(1-4):384–391. doi: 10.1159/000231705. [DOI] [PubMed] [Google Scholar]
- Lester E. P., Miller J. B., Yachnin S. Human alpha-fetoprotein as a modulator of human lymphocyte transformation: correlation of biological potency with electrophoretic variants. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4645–4648. doi: 10.1073/pnas.73.12.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse J. H. Immunological studies of phytohaemagglutinin. I. Reaction between phytohaemagglutinin and normal sera. Immunology. 1968 May;14(5):713–724. [PMC free article] [PubMed] [Google Scholar]
- Morse J. H., Stearns G., Arden J., Agosto G. M., Canfield R. E. The effects of crude and purified human gonadotropin on in vitro stimulated human lymphocyte cultures. Cell Immunol. 1976 Aug;25(2):178–188. doi: 10.1016/0008-8749(76)90108-8. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Johnson B. M. Ontogeny of mouse lymphocyte function. II. Development of the ability to produce antibody is modulated by T lymphocytes. J Exp Med. 1975 Jan 1;141(1):216–226. doi: 10.1084/jem.141.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchmore A. V., Blaese R. M. Immunoregulatory properties of fractions from human pregnancy urine: evidence that human chorionic gonadotropin is not responsible. J Immunol. 1977 Mar;118(3):881–886. [PubMed] [Google Scholar]
- Murgita R. A., Goidl E. A., Kontianen S., Wigzell H. alpha-Fetoprotein induces suppressor T cells in vitro. Nature. 1977 May 19;267(5608):257–259. doi: 10.1038/267257a0. [DOI] [PubMed] [Google Scholar]
- Murgita R. A. The immunosuppressive role of alpha-fetoprotein during pregnancy. Scand J Immunol. 1976;5(9):1003–1014. doi: 10.1111/j.1365-3083.1976.tb03052.x. [DOI] [PubMed] [Google Scholar]
- Murgita R. A., Tomasi T. B., Jr Suppression of the immune response by alpha-fetoprotein on the primary and secondary antibody response. J Exp Med. 1975 Feb 1;141(2):269–286. doi: 10.1084/jem.141.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez E., Vallette G., Benassayag C., Jayle M. F. Comparative study on the binding of estrogens by human and rat serum proteins in development. Biochem Biophys Res Commun. 1974 Mar 15;57(1):126–133. doi: 10.1016/s0006-291x(74)80366-9. [DOI] [PubMed] [Google Scholar]
- Occhino J. C., Glasgow A. H., Cooperband S. R., Mannick J. A., Schmid K. Isolation of an immunosuppressive peptide fraction from human plasma. J Immunol. 1973 Mar;110(3):685–694. [PubMed] [Google Scholar]
- Olding L. B., Murgita R. A., Wigzell H. Mitogen-stimulated lymphoid cells from human newborns suppress the proliferation of maternal lymphocytes actoss a cell-impermeable membrane. J Immunol. 1977 Sep;119(3):1109–1114. [PubMed] [Google Scholar]
- Olding L. B., Oldstone B. A. Thymus-derived peripheral lymphocytes from human newborns inhibit division of their mothers' lymphocytes. J Immunol. 1976 Mar;116(3):682–686. [PubMed] [Google Scholar]
- Ruoslahti E., Seppälä M. Studies of carcino-fetal proteins. 3. Development of a radioimmunoassay for -fetoprotein. Demonstration of -fetoprotein in serum of healthy human adults. Int J Cancer. 1971 Nov 15;8(3):374–383. doi: 10.1002/ijc.2910080304. [DOI] [PubMed] [Google Scholar]
- Sasaki M., Sekizawa T., Takahashi H., Abo T., Kumagai K. Heterogeneity of human T lymphocytes to bind sheep erythrocytes and mitogenic responses of their subpopulations. J Immunol. 1975 Dec;115(6):1509–1514. [PubMed] [Google Scholar]
- Schiff R. I., Mercier D., Buckley R. H. Inability of gestational hormones to account for the inhibitory effects of pregnancy plasmas on lymphocyte responses in vitro. Cell Immunol. 1975 Nov;20(1):69–80. doi: 10.1016/0008-8749(75)90085-4. [DOI] [PubMed] [Google Scholar]
- Seppälä M., Ruoslahti E. Radioimmunoassay of maternal serum alpha fetoprotein during pregnancy and delivery. Am J Obstet Gynecol. 1972 Jan 15;112(2):208–212. doi: 10.1016/0002-9378(72)90117-2. [DOI] [PubMed] [Google Scholar]
- Stobo J. D., Paul W. E. Functional heterogeneity of murine lymphoid cells. 3. Differential responsiveness of T cells to phytohemagglutinin and concanavalin A as a probe for T cell subsets. J Immunol. 1973 Feb;110(2):362–375. [PubMed] [Google Scholar]
- Wolf R. L., Lominitzer R., Rabson A. R. An inhibitor of lymphocyte proliferation and lymphokine production released by unstimulated foetal monocytes. Clin Exp Immunol. 1977 Mar;27(3):464–468. [PMC free article] [PubMed] [Google Scholar]
- Yachnin S., Lester E. Inhibition of human lymphocyte transformation by human alpha-foetoprotein (HAFP); comparison of foetal and hepatoma HAFP and kinetic studies in vitro immunosuppression. Clin Exp Immunol. 1976 Dec;26(3):484–490. [PMC free article] [PubMed] [Google Scholar]
- Zimmerman E. F., Voorting-Hawking M., Michael J. G. Immunosuppression by mouse sialylated alpha-foetoprotein. Nature. 1977 Jan 27;265(5592):354–356. doi: 10.1038/265354a0. [DOI] [PubMed] [Google Scholar]


