Abstract
Studies have been made of the idiotypic determinants of subacute sclerosing panencephalitis (SSPE) antibodies using rabbit antisera to serum and spinal fluid fractions.Evidence is presented indicating that serum and cerebrospinal fluid (CSF) anti-measles antibodies, as judged by their idiotypes, differ in their relative concentrations in the two compartments. The results indicate that some of these antibody subpopulations originate within the CNS, while others are made largely or entirely outside. In addition to strong idiotypic specificity, a limited cross-idiotypic specificity relating antibodies from three out of fourteen SSPE patients has been identified. In the course of these studies, measles virus was found to agglutinate red cells coated with antibody fraction to high titres. This system has proved useful in demonstrating the competition between anti-idiotypic antibody and antigen for the combining sites of the measles antibody. Two anti-idiotypic antisera have also been obtained against the spinal fluid IgG of multiple sclerosis (MS) patients. The possible use of these marker reagnets as well as related methodologies in the search for the antigens involved in MS bands is discussed.
Full text
PDF

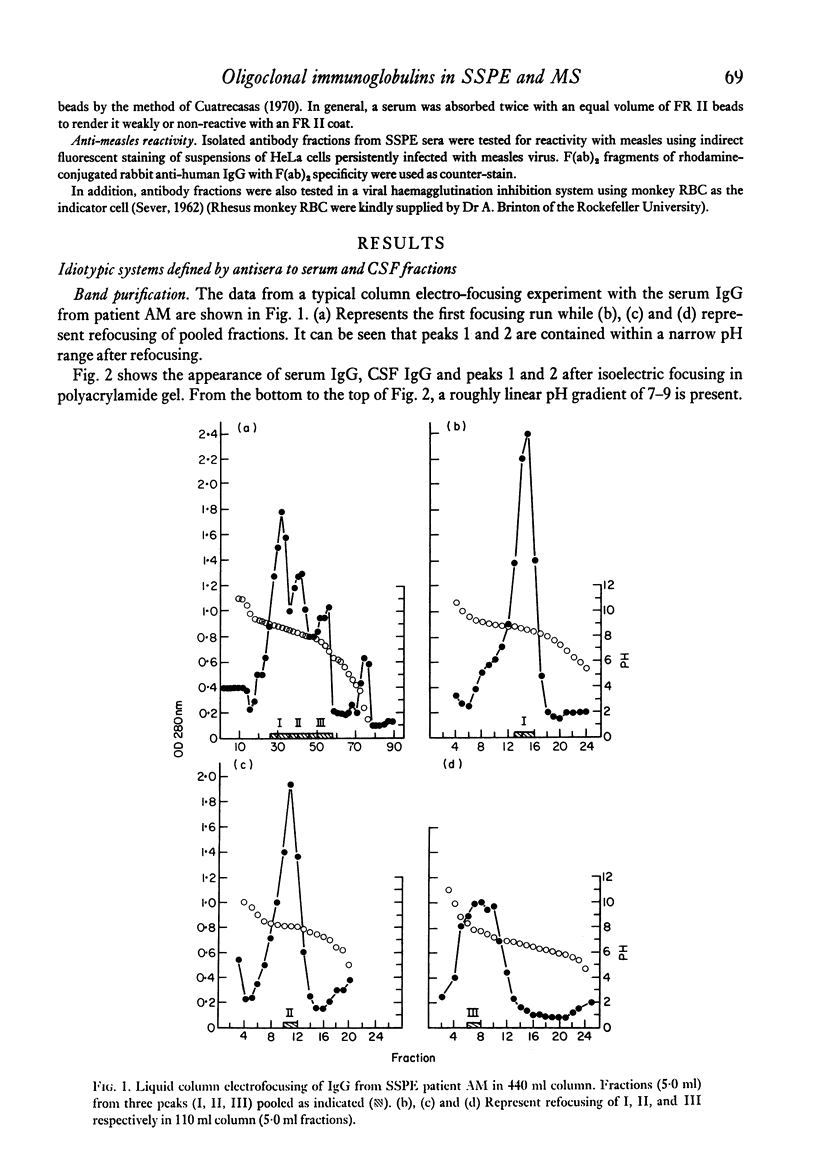

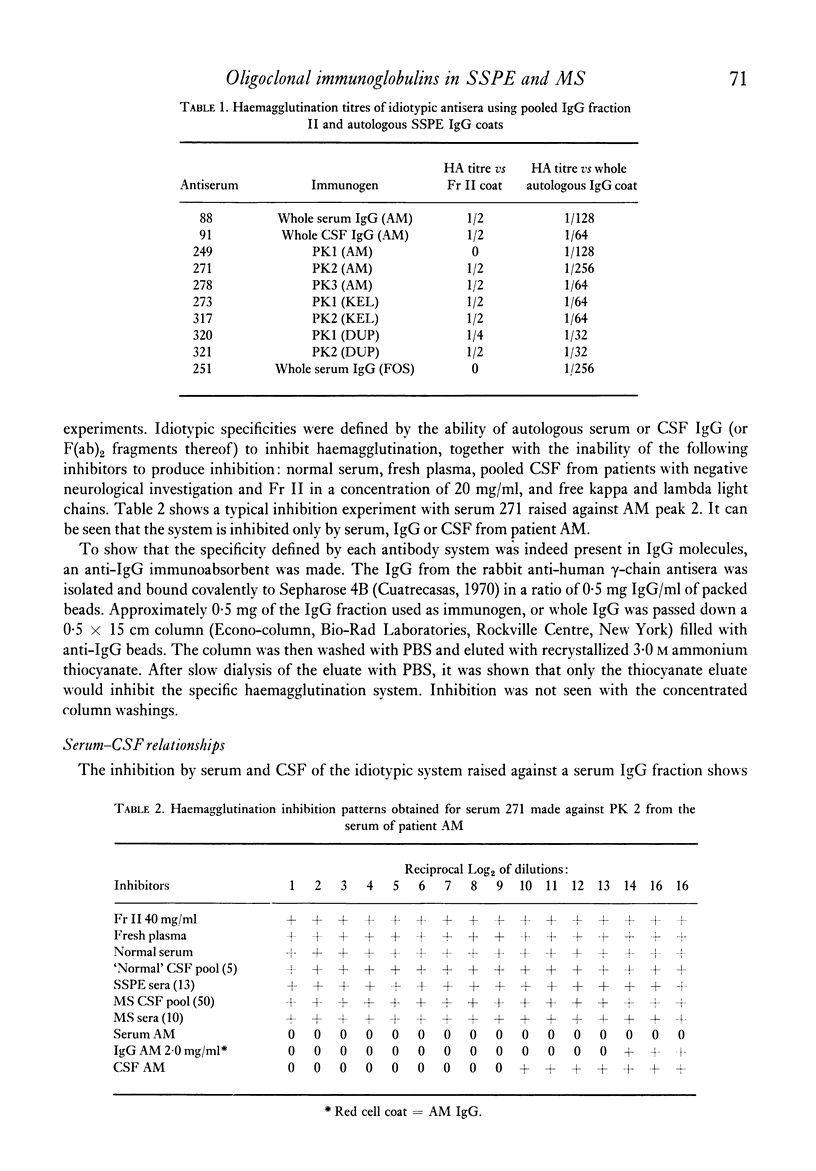
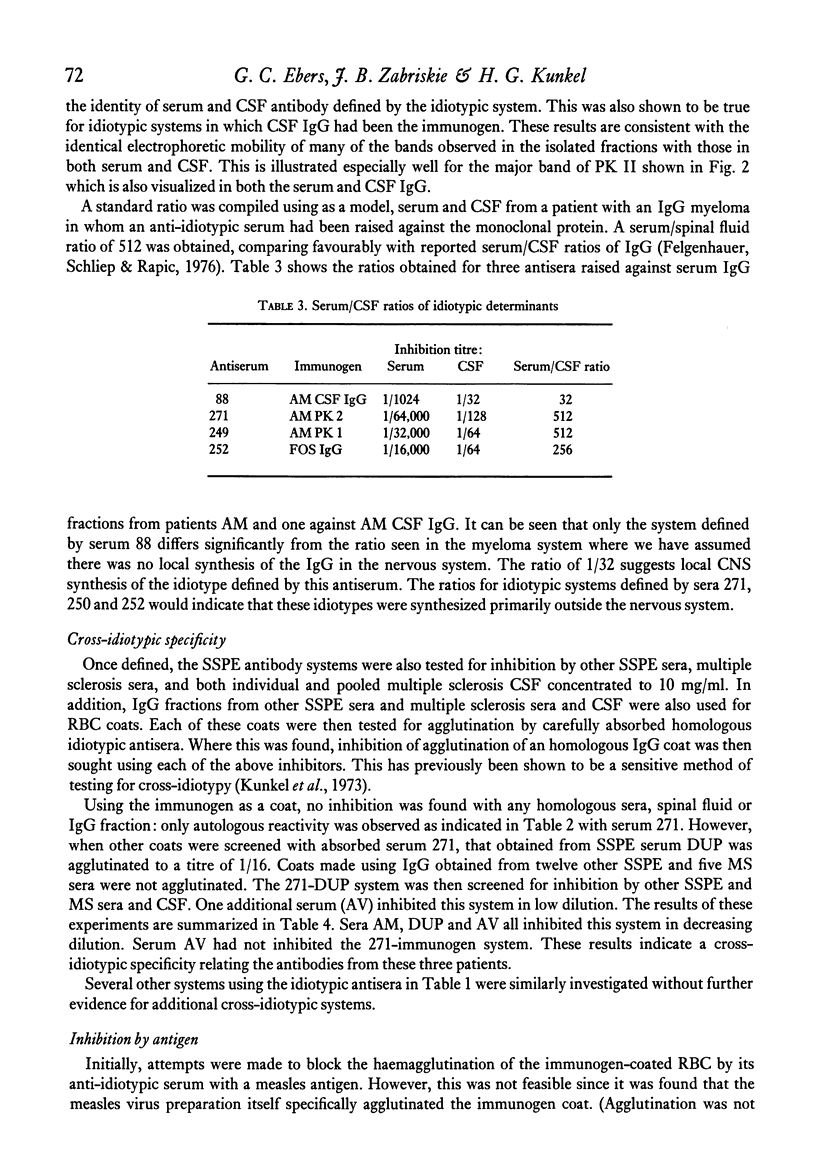



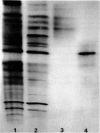
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Awdeh Z. L., Williamson A. R., Askonas B. A. One cell-one immunoglobulin. Origin of limited heterogeneity of myeloma proteins. Biochem J. 1970 Jan;116(2):241–248. doi: 10.1042/bj1160241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollengier F., Lowenthal A., Henrotin W. Bound and free light chains in subacute sclerosing panencephalitis and multiple sclerosis serum and cerebrospinal fluid. Z Klin Chem Klin Biochem. 1975 Jul;13(7):305–310. doi: 10.1515/cclm.1975.13.7.305. [DOI] [PubMed] [Google Scholar]
- Braun D. G., Eichmann K., Krause R. M. Rabbit antibodies to streptococcal carbohydrates. Influence of primary and secondary immunization and of possible genetic factors on the antibody response. J Exp Med. 1969 Apr 1;129(4):809–830. doi: 10.1084/jem.129.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly J. H., Allen I. V., Hurwitz L. J., Millar J. H. Measles-virus antibody and antigen in subacute sclerosing panencephalitis. Lancet. 1967 Mar 11;1(7489):542–544. doi: 10.1016/s0140-6736(67)92117-4. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Felgenhauer K., Schliep G., Rapic N. Evaluation of the blood-CSF barrier by protein gradients and the humoral immune response within the central nervous system. J Neurol Sci. 1976 Nov;30(1):113–128. doi: 10.1016/0022-510x(76)90259-8. [DOI] [PubMed] [Google Scholar]
- Forre O., Natvig J. B., Michaelsen T. E. Cross-idiotypic reactions among anti-Rh (D) antibodies. Scand J Immunol. 1977;6(10):997–1003. doi: 10.1111/j.1365-3083.1977.tb00335.x. [DOI] [PubMed] [Google Scholar]
- GORDON J., ROSE B., SEHON A. H. Detection of non-precipitating antibodies in sera of individuals allergic to ragweed pollen by an in vitro method. J Exp Med. 1958 Jul 1;108(1):37–51. doi: 10.1084/jem.108.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horta-Barbosa L., Hamilton R., Wittig B., Fuccillo D. A., Sever J. L., Vernon M. L. Subacute sclerosing panencephalitis: isolation of suppressed measles virus from lymph node biopsies. Science. 1971 Aug 27;173(3999):840–841. doi: 10.1126/science.173.3999.840. [DOI] [PubMed] [Google Scholar]
- Johnson K. P., Arrigo S. C., Nelson B. J., Ginsberg A. Agarose electrophoresis of cerebrospinal fluid in multiple sclerosis. A simplified method for demonstrating cerebrospinal fluid oligoclonal immunoglobulin bands. Neurology. 1977 Mar;27(3):273–277. doi: 10.1212/wnl.27.3.273. [DOI] [PubMed] [Google Scholar]
- KUNKEL H. G. Zone electrophoresis. Methods Biochem Anal. 1954;1:141–170. doi: 10.1002/9780470110171.ch6. [DOI] [PubMed] [Google Scholar]
- Kunkel H. G., Agnello V., Joslin F. G., Winchester R. J., Capra J. D. Cross-idiotypic specificity among monoclonal IgM proteins with anti- -globulin activity. J Exp Med. 1973 Feb 1;137(2):331–342. doi: 10.1084/jem.137.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel H. G., Mannik M., Williams R. C. Individual Antigenic Specificity of Isolated Antibodies. Science. 1963 Jun 14;140(3572):1218–1219. doi: 10.1126/science.140.3572.1218. [DOI] [PubMed] [Google Scholar]
- Nodal H. J., Vandvik B., Natvig J. B. Idiotypy of measles virus nucleocapsid-specific IgGkappa antibody in serum and cerebrospinal fluid in subacute sclerosing panencephalitis. Scand J Immunol. 1977;6(12):1351–1356. doi: 10.1111/j.1365-3083.1977.tb00377.x. [DOI] [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathnam P., Saxena B. B. Isolation and physicochemical characterization of luteinizing hormone from human pituitary glands. J Biol Chem. 1970 Jul 25;245(14):3725–3731. [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Salmi A. A., Norrby E., Panelius M. Identification of different measles virus-specific antibodies in the serum and cerebrospinal fluid from patients with subacute sclerosing pancencephalitis and multiple sclerosis. Infect Immun. 1972 Sep;6(3):248–254. doi: 10.1128/iai.6.3.248-254.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbuch M., Audran R. The isolation of IgG from mammalian sera with the aid of caprylic acid. Arch Biochem Biophys. 1969 Nov;134(2):279–284. doi: 10.1016/0003-9861(69)90285-9. [DOI] [PubMed] [Google Scholar]
- Stejskal V. Differential cytotoxicity of activated lymphocytes on allogeneic and xenogeneic target cells. IV. Competitive inhibition of target cell lysis by addition of unlabeled cells. Scand J Immunol. 1976;5(5):479–486. doi: 10.1111/j.1365-3083.1976.tb00302.x. [DOI] [PubMed] [Google Scholar]
- Vandvik B., Natvig J. B., Norrby E. IgG1 subclass restriction of oligoclonal measles virus-specific IgG antibodies in patients with subacute sclerosing panencephalitis and in a patient with multiple sclerosis. Scand J Immunol. 1977;6(6-7):651–657. doi: 10.1111/j.1365-3083.1977.tb02145.x. [DOI] [PubMed] [Google Scholar]
- Vandvik B., Natvig J. B., Wiger D. IgG1 subclass restriction of oligoclonal IgG from cerebrospinal fluids and brain extracts in patients with multiple sclerosis and subacute encephalitides. Scand J Immunol. 1976;5(4):427–436. doi: 10.1111/j.1365-3083.1976.tb00297.x. [DOI] [PubMed] [Google Scholar]
- Vandvik B., Norrby E. Oligoclonal IgG antibody response in the central nervous system to different measles virus antigens in subacute sclerosing panencephalitis. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1060–1063. doi: 10.1073/pnas.70.4.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willims R. C., Jr, Kunkel H. G., Capra J. D. Antigenic specificities related to the cold agglutinin activity of gamma M globulins. Science. 1968 Jul 26;161(3839):379–381. doi: 10.1126/science.161.3839.379. [DOI] [PubMed] [Google Scholar]