Abstract
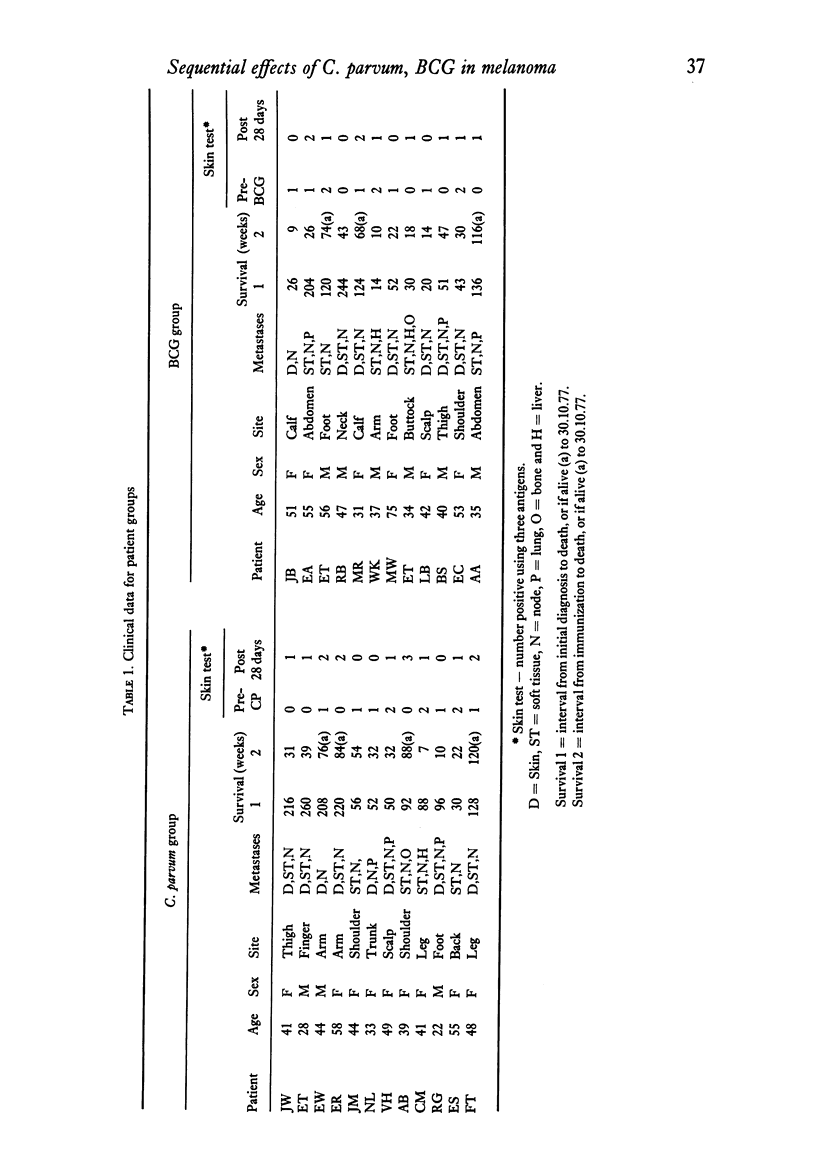
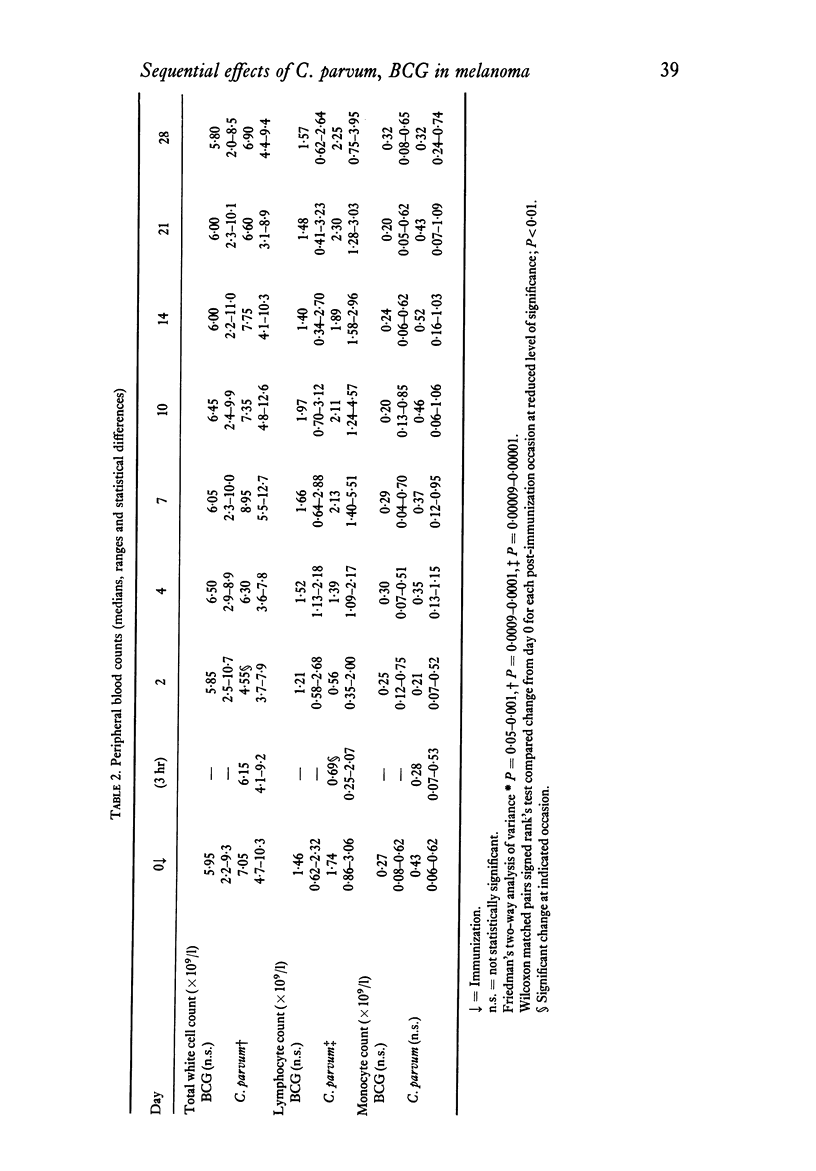
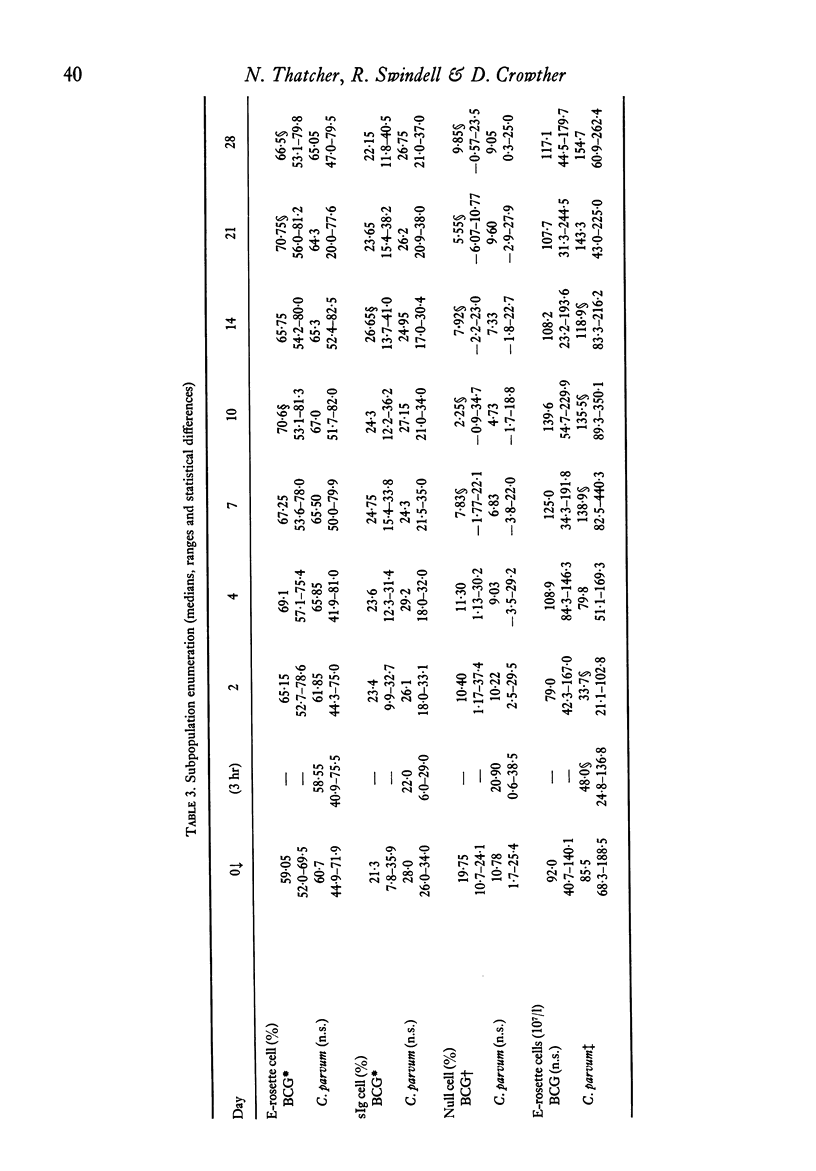
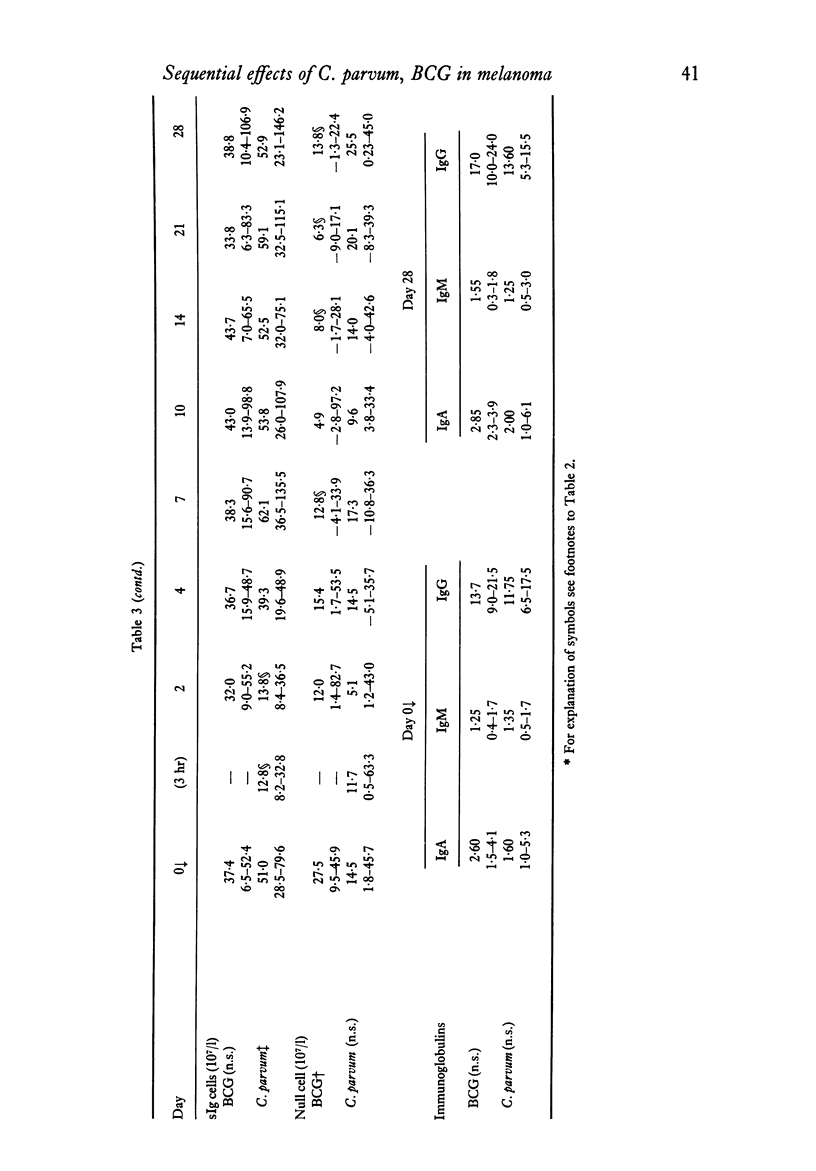
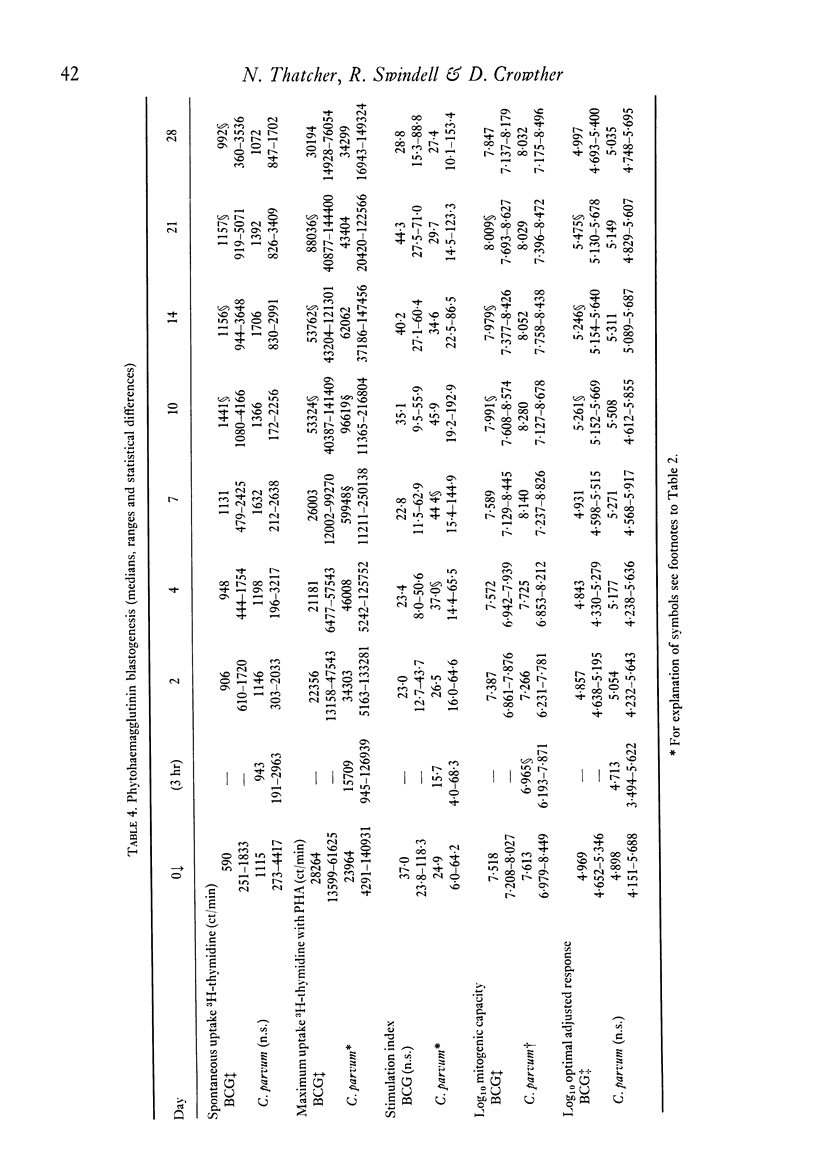
The effects of a single immunization of melanoma patients with BCG or C. parvum on the blood counts, serum immunoglobulin levels and lymphoid subpopulations were followed by multiple assays over 28 days. C. parvum produced a decrease in the white cell count, lymphocyte count and lymphoid T and sIg+ cell numbers, which recovered within 1 week; BCG did not produce such a marked depression. Both agents were associated with increases in T cell numbers and lymphocyte PHA blastogenesis after the first week; these declined to pre-immunization values by 3-4 weeks. The sIg-bearing cell subpopulation also increased after BCG. Different methods of expression the results were compared and the difficulties of immunological monitoring are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell A. C., Hersey P., MacLennan I. C., Kay H. E., Pike M. C. Immunosuppressive consequences of radiotherapy and chemotherapy in patients with acute lymphoblastic leukaemia. Br Med J. 1973 May 19;2(5863):385–388. doi: 10.1136/bmj.2.5863.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub S. H., Forsythe A. B., Morton D. L. Sequential examination of lymphocyte proliferative capacity in patients with malignant melanoma receiving BCG immunotherapy. Int J Cancer. 1977 Jan;19(1):18–26. doi: 10.1002/ijc.2910190104. [DOI] [PubMed] [Google Scholar]
- Golub S. H., O'Connell T. X., Morton D. L. Correlation of in vivo and in vitro assays of immunocompetence in cancer patients. Cancer Res. 1974 Aug;34(8):1833–1837. [PubMed] [Google Scholar]
- Gross N. J., Eddie-Quartey A. C. Monitoring of immunologic status of patients receiving BCG therapy for malignant disease. Cancer. 1976 May;37(5):2183–2193. doi: 10.1002/1097-0142(197605)37:5<2183::aid-cncr2820370505>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Kehlet H., Thomsen M., Kjaer M., Platz P. Postoperative depression of lymphocyte transformation response to microbial antigens. Br J Surg. 1977 Dec;64(12):890–893. doi: 10.1002/bjs.1800641215. [DOI] [PubMed] [Google Scholar]
- Minton J. P., Rossio J. L., Dixon B., Dodd M. C. The effect of Corynebacterium parvum on the humoral and cellular immune systems in patients with breast cancer. Clin Exp Immunol. 1976 Jun;24(3):441–447. [PMC free article] [PubMed] [Google Scholar]
- Potter M. R., Moore M. The effect of adherent and phagocytic cells on human lymphocyte PHA responsiveness. Clin Exp Immunol. 1977 Jan;27(1):159–164. [PMC free article] [PubMed] [Google Scholar]
- Slade M. S., Simmons R. L., Yunis E., Greenberg L. J. Immunodepression after major surgery in normal patients. Surgery. 1975 Sep;78(3):363–372. [PubMed] [Google Scholar]
- Thatcher N., Palmer M. K., Gasiunas N., Crowther D. Lymphocyte function and response to chemo-immunotherapy in patients with metastatic melanoma. Br J Cancer. 1977 Dec;36(6):751–762. doi: 10.1038/bjc.1977.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weese J. L., Herberman R. B., Perlin E., Mills M., Heims W., Blom J., Green D., Reid J., Bellinger S., Law I. Immunological monitoring and immunotherapy in carcinoma of the lung. Int J Cancer. 1976 Dec 15;18(6):739–749. doi: 10.1002/ijc.2910180604. [DOI] [PubMed] [Google Scholar]


