Abstract
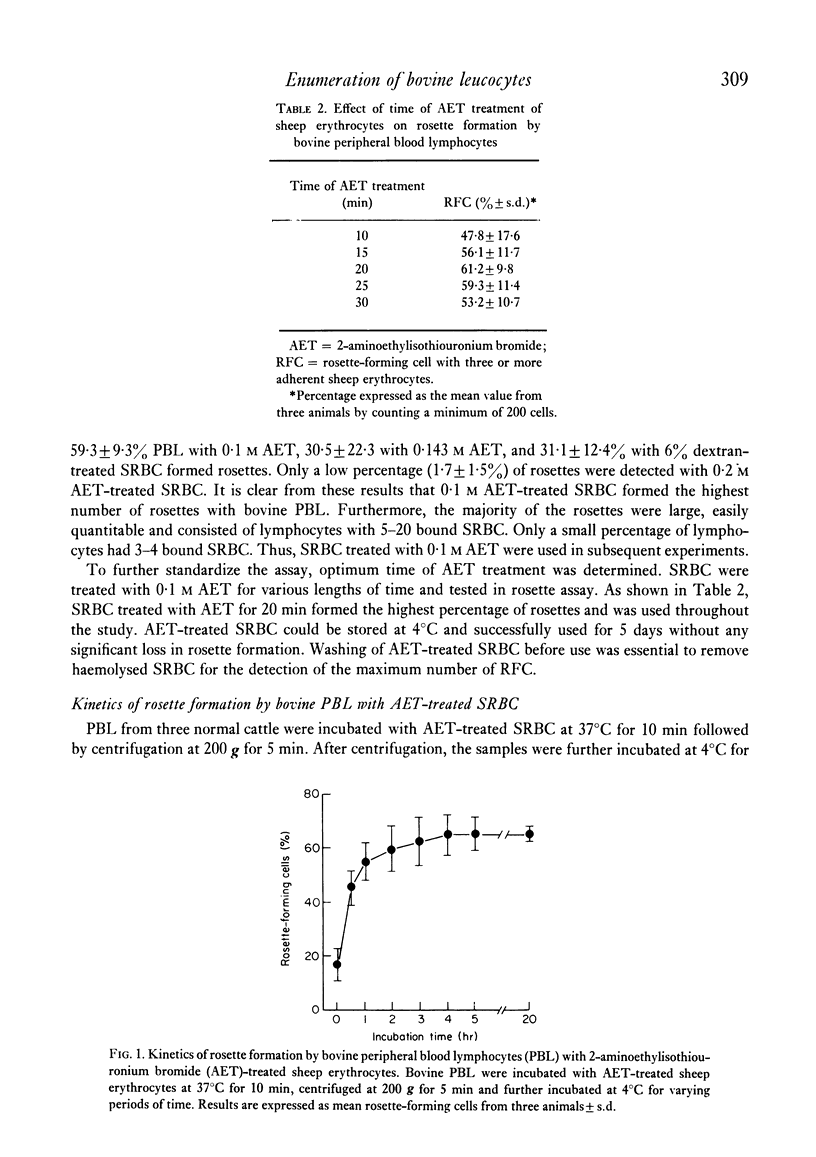
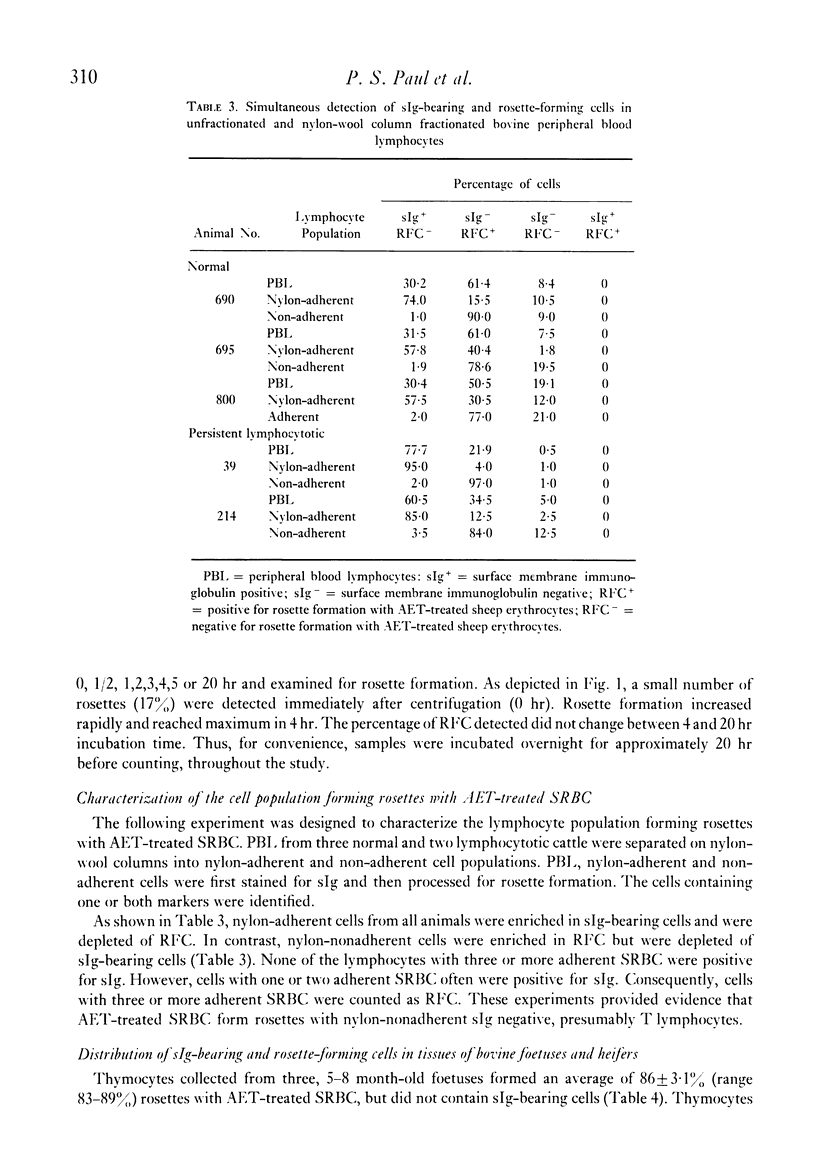
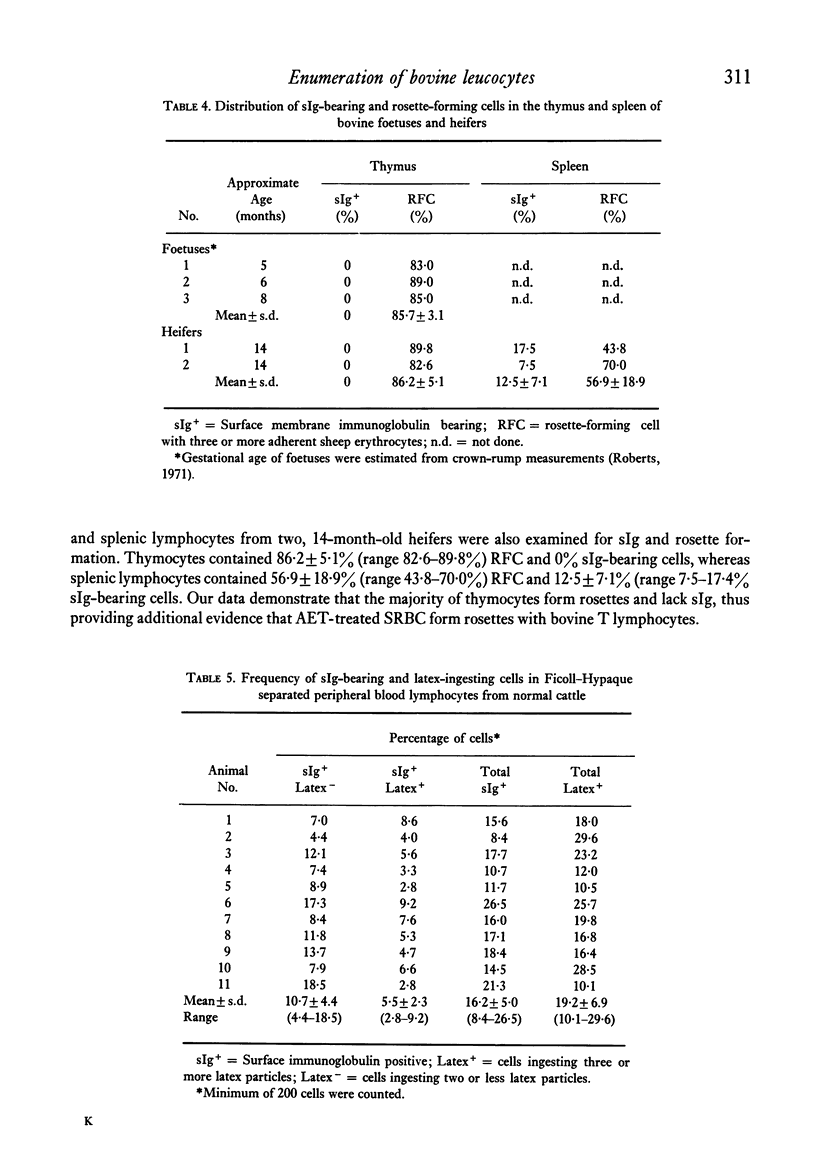
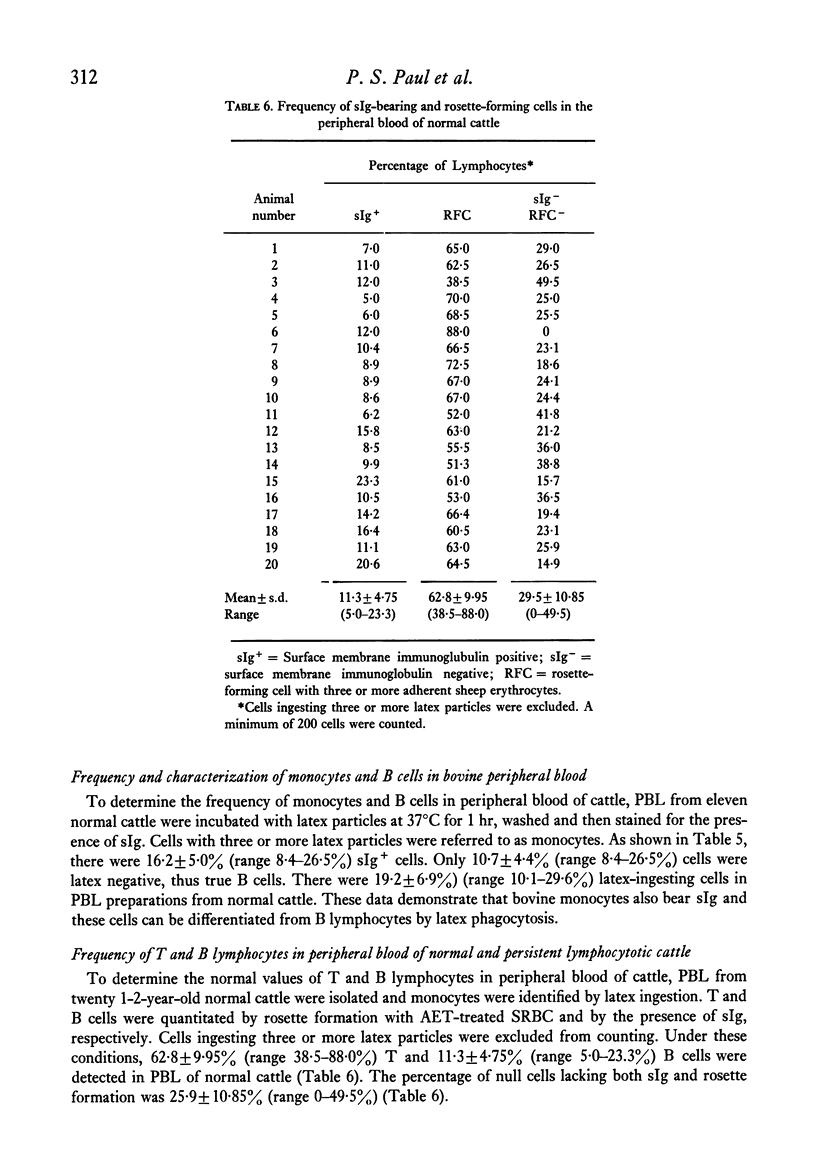
The rosette-forming capacity of bovine peripheral blood lymphocytes (PBL) was determined with dextran and 2-aminoethylisothiouronium bromide (AET)-treated sheep erythrocytes (SRBC). Both dextran and AET-enhanced rosette formation; however, AET-treated SRBC detected a larger percentage of rosette-forming cells and thus was used in this study. The specificity of rosette formation by bovine thymus-derived (T) lymphocytes was shown by (1) demonstration of rosettes and surface-membrane immunoglobulins sIg) on different cells in PBL and nylon-wool fractionated lymphocyte populations and (2) rosette formation by a large percentage (83--90%) of thymocytes from three bovine foetuses and two 14-month-old heifers. A procedure was also developed to identify bovine monocytes by latex phagocytosis and 10--30% latex-ingesting cells were detected in PBL preparations isolated by Ficoll-Hypaque flotation. The frequency of sIg-bearing latex-ingesting, and sIg-bearing latex non-ingesting cells in bovine peripheral blood was also determined. These procedures were utilized to determine the distribution of T and bone-marrow derived (B) lymphocytes in peripheral blood of normal and lymphocytotic cattle. PBL from twenty normal cattle contained approximately 63% T and 11% B (sIg+ latex non-ingesting) lymphocytes. In peripheral blood of three cattle with persistent lymphocytosis, a prodromal stage of bovine leukaemia, the percentage of B cells was elevated approximately to 59% whereas T lymphocytes decreased to 35%, thus providing additional evidence that persistent lymphocytosis is a B-cell disease.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach J. F., Dormont J., Dardenne M., Balner H. In vitro rosette inhibition by antihuman antilymphocyte serum. Correlation with skin graft prolongation in subhuman primates. Transplantation. 1969 Sep;8(3):265–280. doi: 10.1097/00007890-196909000-00008. [DOI] [PubMed] [Google Scholar]
- Bowles C. A., White G. S., Lucas D. Rosette formation by canine peripheral blood lymphocytes. J Immunol. 1975 Jan;114(1 Pt 2):399–402. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cockerell G. L., Krakowka S., Hoover E. A., Olsen R. G., Yohn D. S. Characterization of feline T-and B-lymphocytes and identification of an experimentally induced T-cell neoplasm in the cat. J Natl Cancer Inst. 1976 Oct;57(4):907–913. doi: 10.1093/jnci/57.4.907. [DOI] [PubMed] [Google Scholar]
- Escajadillo C., Binns R. M. Rosette formation of pig T lymphocytes with sheep erythrocytes. Int Arch Allergy Appl Immunol. 1975;49(3):325–331. doi: 10.1159/000231413. [DOI] [PubMed] [Google Scholar]
- Escajadillo C., Binns R. M. Rosette formation with sheep erythrocytes--a possible T-cell marker in the pig. Int Arch Allergy Appl Immunol. 1975;48(2):261–275. doi: 10.1159/000231312. [DOI] [PubMed] [Google Scholar]
- Florey M. J., Peetoom F. Modified E-rosette test for detection of total and active rosette-forming lymphocytes. J Immunol Methods. 1976;13(3-4):201–206. doi: 10.1016/0022-1759(76)90066-1. [DOI] [PubMed] [Google Scholar]
- Greenberg R. S., Zatz M. M. Spontaneous AKR lymphoma with T and B-cell characteristics. Nature. 1975 Sep 25;257(5524):314–316. doi: 10.1038/257314a0. [DOI] [PubMed] [Google Scholar]
- Grewal A. S., Rouse B. T., Babiuk L. A. Characterization of surface receptors on bovine leukocytes. Int Arch Allergy Appl Immunol. 1978;56(4):289–300. doi: 10.1159/000232034. [DOI] [PubMed] [Google Scholar]
- Grewal A. S., Rouse B. T., Babiuk L. A. Erythrocyte rosettes--a marker for bovine T cells. Can J Comp Med. 1976 Jul;40(3):298–305. [PMC free article] [PubMed] [Google Scholar]
- Higgins D. A., Stack M. J. Bovine lymphocytes: recognition of cells forming spontaneous (E) rosettes. Clin Exp Immunol. 1977 Feb;27(2):348–356. [PMC free article] [PubMed] [Google Scholar]
- Horwitz D. A., Allison A. C., Ward P., Kight N. Identification of human mononuclear leucocyte populations by esterase staining. Clin Exp Immunol. 1977 Nov;30(2):289–298. [PMC free article] [PubMed] [Google Scholar]
- Johansen K. S., Johansen T. S., Talmage D. W. T cell rosette formation in primates, pigs, and guinea pigs. The influence of immunosuppresive agents. J Allergy Clin Immunol. 1974 Aug;54(2):86–93. doi: 10.1016/0091-6749(74)90036-0. [DOI] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. E., Clark C. An improved rosetting assay for detection of human T lymphocytes. J Immunol Methods. 1974 Jul;5(2):131–135. doi: 10.1016/0022-1759(74)90003-9. [DOI] [PubMed] [Google Scholar]
- Kenyon S. J., Piper C. E. Cellular basis of persistent lymphocytosis in cattle infected with bovine leukemia virus. Infect Immun. 1977 Jun;16(3):891–897. doi: 10.1128/iai.16.3.891-897.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S. P., Paul P. S., Pomeroy K. A., Johnson D. W., Muscoplat C. C., Van Der Maaten M. J., Miller J. M., Sorensen D. K. Frequency of lymphocytes bearing Fc receptors and surface membrane immunoglobulins in normal, persistent lymphocytotic and leukemia cows. Am J Vet Res. 1978 Jan;39(1):45–49. [PubMed] [Google Scholar]
- Lay W. H., Mendes N. F., Bianco C., Nussenzweig V. Binding of sheep red blood cells to a large population of human lymphocytes. Nature. 1971 Apr 23;230(5295):531–532. doi: 10.1038/230531a0. [DOI] [PubMed] [Google Scholar]
- Paul P. S., Pomeroy K. A., Castro A. E., Johnson D. W., Muscoplat C. C., Sorensen D. K. Detection of bovine leukemia virus in B-lymphocytes by the syncytia induction assay. J Natl Cancer Inst. 1977 Oct;59(4):1269–1272. doi: 10.1093/jnci/59.4.1269. [DOI] [PubMed] [Google Scholar]
- Paul P. S., Pomeroy K. A., Johnson D. W., Muscoplat C. C., Handwerger B. S., Soper F. F., Sorensen D. K. Evidence for the replication of bovine leukemia virus in the B lymphocytes. Am J Vet Res. 1977 Jun;38(6):873–876. [PubMed] [Google Scholar]
- Pellegrino M. A., Ferrone S., Dierich M. P., Reisfeld R. A. Enhancement of sheep red blood cell human lymphocyte rosette formation by the sulfhydryl compound 2-amino ethylisothiouronium bromide. Clin Immunol Immunopathol. 1975 Jan;3(3):324–333. doi: 10.1016/0090-1229(75)90019-7. [DOI] [PubMed] [Google Scholar]
- Preud'homme J. L., Flandrin G. Identification by peroxidase staining of monocytes in surface immunofluorescence tests. J Immunol. 1974 Nov;113(5):1650–1653. [PubMed] [Google Scholar]
- Preud'homme J. L., Seligmann M. Surface immunoglobulins on human lymphoid cells. Prog Clin Immunol. 1974;2:121–174. [PubMed] [Google Scholar]
- Rouse B. T., Babiuk L. A. Host responses to infectious bovine rhinotracheitis virus. III. Isolation and immunologic activities of bovine T lymphocytes. J Immunol. 1974 Nov;113(5):1391–1398. [PubMed] [Google Scholar]
- Shimizu M., Pan I. C., Hess W. R. T and B lymphocytes in porcine blood. Am J Vet Res. 1976 Mar;37(3):309–317. [PubMed] [Google Scholar]
- Tarr M. J., Olsen R. G., Krakowka G. S., Cockerell G. L., Gabel A. A. Erythrocyte rosette formation of equine peripheral blood lymphocytes. Am J Vet Res. 1977 Nov;38(11):1775–1779. [PubMed] [Google Scholar]
- Terrell T. G., Holmberg C. A., Osburn B. I. Immunologic surface markers on nonhuman primate lymphocytes. Am J Vet Res. 1977 Apr;38(4):503–507. [PubMed] [Google Scholar]
- Townes A. S., Postlethwaite A. E. Lymphocyte surface markers in human disease. Adv Intern Med. 1977;22:97–119. [PubMed] [Google Scholar]
- Wardley R. An improved E rosetting technique for cattle. Br Vet J. 1977 Jul-Aug;133(4):432–434. doi: 10.1016/s0007-1935(17)34047-2. [DOI] [PubMed] [Google Scholar]
- Weiland F., Straub O. C. Frequency of surface immunoglobulin bearing blood lymphocytes in cattle affected with bovine leukosis. Res Vet Sci. 1975 Jul;19(1):100–102. [PubMed] [Google Scholar]
- Weiner M. S., Bianco C., Nussenzweig V. Enhanced binding of neuraminidase-treated sheep erythrocytes to human T lymphocytes. Blood. 1973 Dec;42(6):939–946. [PubMed] [Google Scholar]
- Woody J. N., Sell K. W. Characteristics of the 'active rosette test'. I. Technical considerations of the test and comments. J Immunol Methods. 1975 Oct;8(4):331–338. doi: 10.1016/0022-1759(75)90054-x. [DOI] [PubMed] [Google Scholar]
- Wybran J., Fudenberg H. H. Thymus-derived rosette-forming cells in various human disease states: cancer, lymphoma, bacterial and viral infections, and other diseases. J Clin Invest. 1973 May;52(5):1026–1032. doi: 10.1172/JCI107267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker-Franklin D. The percentage of monocytes among "mononuclear" cell fractions obtained from normal human blood. J Immunol. 1974 Jan;112(1):234–240. [PubMed] [Google Scholar]


